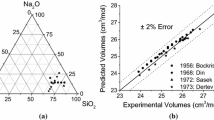
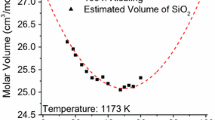
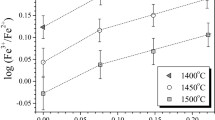
Abstract
As part III of this series, the model is extended to iron oxide-containing melts. All available experimental data in the FeO-Fe2O3-Na2O-K2O-MgO-CaO-MnO-Al2O3-SiO2 system were critically evaluated based on the experimental condition. The variations of FeO and Fe2O3 in the melts were taken into account by using FactSage to calculate the Fe2+/Fe3+ distribution. The molar volume model with unary and binary model parameters can be used to predict the molar volume of the molten oxide of the Li2O-Na2O-K2O-MgO-CaO-MnO-PbO-FeO-Fe2O3-Al2O3-SiO2 system in the entire range of compositions, temperatures, and oxygen partial pressures from Fe saturation to 1 atm pressure.






















Similar content being viewed by others
References
E. Thibodeau, A.E. Gheribi, and I.-H. Jung: “Part I,” accepted.
E. Thibodeau, A.E. Gheribi, and I.-H. Jung: “Part II,” accepted.
A. D. Pelton and M. Blander, “Thermodynamic analysis of ordered liquid solutions by a modified quasichemical approach - application to silicate slags,” Metallurgical and Materials Transactions B, vol. 17, pp. 805-815 (1986).
C. W. Bale, E. Bélisle, P. Chartrand, S. A. Decterov, G. Eriksson, K. Hack, I. H. Jung, Y. B. Kang, J. Melançon, A. D. Pelton, C. Robelin, and S. Petersen, “FactSage thermochemical software and databases— recent developments,” Calphad, vol. 33, pp. 295-311 (2009).
R. L. Lange and I. S. E. Carmichael, “Thermodynamic properties of silicate liquids with emphasis on density, thermal expansion and compressibility,” Reviews in Mineralogy and Geochemistry, vol. 24, pp. 25-64 (1990).
S. A. Decterov, E. Jak, P. C. Hayes, and A. D. Pelton, “Experimental study of phase equilibria and thermodynamic optimization of the Fe-Zn-O system,” Metallurgical and Materials Transactions B, vol. 32B, pp. 643-657 (2001).
E. Jak, P. Hayes, A. Pelton, and S. Decterov, “Thermodynamic optimisation of the FeO-Fe2O3-SiO2 (Fe-O-Si) system with FactSage,” International Journal of Materials Research, vol. 98, pp. 847-854 (2007).
I.-H. Jung, S. A. Decterov, and A. D. Pelton, “Critical thermodynamic evaluation and optimization of the Fe-Mg-O system,” Journal of Physics and Chemistry of Solids, vol. 65, pp. 1683-1695 (2004).
S. Decterov, I. H. Jung, E. Jak, Y. B. Kang, P. Hayes, and A. Pelton: VII International Conference on Molten Slags, Fluxes and Salts, 2004, pp. 839–49.
I.-H. Jung, S. A. Decterov, and A. D. Pelton, “Critical thermodynamic evaluation and optimization of the FeO-Fe2O3-MgO-SiO2 system,” Metallurgical and Materials Transactions B, vol. 35B, pp. 877-889 (2004).
Y. B. Kang and I. H. Jung: VIII International Conference on Molten Slags, Fluxes and Salt, 2009, pp. 459–71.
12. J. Henderson, R. G. Hudson, R. G. Ward, and G. Derge, “Density of liquid iron silicates,” Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers, vol. 221, pp. 807-811 (1961).
A. Adachi, K. Ogino, and S. Kawasaki, “Measurement of the density of molten FeO-SiO2 slags by the archimedean method,” Technology Reports of the Osaka University, vol. 13, pp. 411-415 (1963).
J. Henderson, “Density of lime-iron oxide-silica melts,” Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers, vol. 230, pp. 501-504 (1964).
R. G. Ward and P. L. Sachdev, “Density of iron oxide-silica melts,” Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers, vol. 233, pp. 1496-1499 (1965).
D. R. Gaskell and R. G. Ward, “Density of iron oxide-silica melts,” Transactions of the Metallurgical Society of AIME, vol. 239, pp. 249-252 (1967).
K. Mori and K. Suzuki, “Density of iron oxide melts in equilibrium with carbon dioxide-carbon monoxide gas mixtures,” Tetsu to Hagane, vol. 54, pp. 1123-1127 (1968).
V. I. Sokolov, S. I. Popel, and O. A. Esin, “Density and molar volume of slags,” Izvestiya Vysshikh Uchebnykh Zavedenii, Chernaya Metallurgiya, vol. 13, pp. 10-15 (1970).
D. R. Gaskell and Y. E. Lee, “Densities and structures of melts in the system calcium oxide-iron(II, III) oxide-silicon dioxide,” Metallurgical Transactions, vol. 5, pp. 853-860 (1974).
K. Ogino, M. Hirano, and A. Adachi, “Density of iron silicate slags,” Technology Reports of the Osaka University, vol. 24, pp. 49-55 (1974).
Y. Kawai, K. Mori, H. Shiraishi, and N. Yamada, “Surface tension and density of FeO-CaO-SiO2 melts,” Tetsu to Hagane, vol. 62, pp. 53-61 (1976).
Y. Shiraishi, K. Ikeda, A. Tamura, and T. Saito, “On the viscosity and density of the molten FeO-SiO2 system,” Transactions of the Japan Institute of Metals, vol. 19, pp. 264-274 (1978).
S. Hara, K. Irie, D. R. Gaskell, and K. Ogino, “Densities of melts in the iron(II) oxide-iron(III) oxide-calcium oxide and iron(II) oxide-iron(III) oxide-dicalcium silicate (2CaO.SiO2) systems,” Transactions of the Japan Institute of Metals, vol. 29, pp. 977-989 (1988).
A. S. Abrosimov, E. G. Gavrin, and V. I. Eremeichenkov, Izvestiya Vysshikh Uchebnykh Zavedenii, Chernaya Metallurgiya, 1975, pp. 14–17.
S. Sumita, K. Morinaga, and T. Yanagase, “Density and surface tension of binary ferrite melts,” Journal of the Japan Institute of Metals, vol. 47, pp. 127-131 (1983).
A. Adachi and K. Ogino, “The density of molten slags containing ferrous oxide,” Technology Reports of the Osaka University, vol. 12, pp. 147-152 (1962).
D. R. Gaskell, A. McLean, and R. G. Ward, “Density and structure of ternary silicate melts,” Transactions of the Faraday Society, vol. 65, pp. 1498-1508 (1969).
J. Holeczy, L. Bodnar, and K. Tomasek, “Density of an iron-oxygen-silica slag system,” Hutnicke Listy, vol. 27, pp. 364-368 (1972).
X. Mo, I. S. E. Carmichael, M. Rivers, and J. Stebbins, “The partial molar volume of iron oxide in multicomponent silicate liquids and the pressure dependence of oxygen fugacity in magmas,” Mineralogical Magazine, vol. 45, pp. 237-245 (1982).
P. Courtial, E. Ohtani, and D. B. Dingwell, “High-temperature densities of some mantle melts,” Geochimica et Cosmochimica Acta, vol. 61, pp. 3111-3119 (1997).
R. A. Lange and I. S. E. Carmichael, “Densities of Na2O-K2O-MgO-MgO-FeO-Fe2O3-Al3O3-TiO2-SiO2 liquids: New measurements and derived partial molar properties,” Geochimica et Cosmochimica Acta, vol. 51, pp. 2931-2946 (1987).
D. B. Dingwell, M. Brearley, and J. E. Dickinson Jr, “Melt densities in the Na2O-FeO-Fe2O3-SiO2 system and the partial molar volume of tetrahedrally-coordinated ferric iron in silicate melts,” Geochimica et Cosmochimica Acta, vol. 52, pp. 2467-2475 (1988).
Q. Liu and R. A. Lange, “The partial molar volume of Fe2O3 in alkali silicate melts: Evidence for an average Fe3+ coordination number near five,” American Mineralogist, vol. 91, pp. 385-393 (2006).
X. Guo: Ph.D. Thesis, University of Michigan, 2013.
M. Zielinski and B. Sikora, “Surface tension of the calcium oxide-alumina system with additions of silica, iron(III) oxide, calcium fluoride, sodium fluoride, and magnesia,” Prace Instytutu Metalurgii Zelaza im. Stanislawa Staszica, vol. 29, pp. 157-165 (1977).
P. Vadász, M. Havlík, and V. Danêk, “Density and surface tension of calcium-ferritic slags I. The systems CaO-FeO-Fe2O3-SiO2 and CaO-FeO-Fe2O3-Al2O3,” Canadian Metallurgical Quarterly, vol. 39, pp. 143-152 (2000).
B. A. Bryantsev, Zhurnal Prikladnoi Khimii, vol. 41, pp. 2156-2159 (1968).
A. Adachi, K. Ogino, and S. Kuno, “The density of molten slags containing ferrous oxide,” Tetsu to Hagane, vol. 46, pp. 1243-1244 (1960).
T.-F. Wu, A. F. Vishkarev, and V. I. Yavoiskii, “Density of molten steel slags,” Izvestiya Vysshikh Uchebnykh Zavedenii, Chernaya Metallurgiya, vol. 5, pp. 66-75 (1962).
T. Licko, V. Danek, and Z. Panek, “Densities of melts in the system calcium oxide-iron(II) oxide-iron(III) oxide-silicon dioxide,” Chemical Papers, vol. 39, pp. 599-605 (1985).
D. B. Dingwell and M. Brearley, “Melt densities in the CaO-FeO-Fe2O3-SiO2 system and the compositional dependence of the partial molar volume of ferric iron in silicate melts,” Geochimica et Cosmochimica Acta, vol. 52, pp. 2815-2825 (1988).
S. A. Nelson and I. S. E. Carmichael, “Partial molar volumes of oxide components in silicate liquids,” Contributions to Mineralogy and Petrology, vol. 71, pp. 117-124 (1979).
P. Vadász, M. Havlík, and V. Daněk, “Density and surface tension of the systems CaO-FeO-Fe2O3-MgO, CaO-FeO-Fe2O3-ZnO and CaO-Fe2O3-Cu2O,” Central European Journal of Chemistry, vol. 4, pp. 174-193 (2006).
C. W. Thomas: Ph.D. Thesis, California Institute of Technology, 2013.
K. Tomasek, L. Bodnar, and J. Holeczy, “Density of iron-oxygen-silicon dioxide slag systems with lime, alumina, magnesia, or zinc oxide and its relation to surface tension,” Hutnicke Listy, vol. 30, pp. 136-138 (1975).
O. N. Fadeev, I. F. Khudyakov, I. D. Kashcheev, and G. P. Kharitidi, “Study of the density and surface tension of ferrous oxide-ferric oxide-silicon dioxide-aluminum oxide system melts,” Izvestiya Vysshikh Uchebnykh Zavedenii, Tsvetnaya Metallurgiya, vol. 1, pp. 17-20 (1980).
K. C. Mills and B. J. Keene, “Physical properties of BOS slags,” International Materials Reviews, vol. 32, pp. 1-120 (1987).
J. O. M. Bockris, J. W. Tomlinson, and J. L. White, “The structure of the liquid silicates: Partial molar volumes and expansivities,” Transactions of the Faraday Society, vol. 52, pp. 299-310 (1956).
J. W. Tomlinson, M. S. R. Heynes, and J. O. M. Bockris, “The structure of liquid silicates. Part 2.-Molar volumes and expansivities,” Transactions of the Faraday Society, vol. 54, pp. 1822-1833 (1958).
M. Hino, T. Ejima, and M. Kameda, “Surface tension and density of liquid lead silicate,” Journal of the Japan Institute of Metals, vol. 31, pp. 113-119 (1967).
51. H. Ito and T. Yanagase, “Studies on lead silicate melts,” Transactions of the Japan Insitute of Melts, vol. 1, pp. 115-120 (1960).
P. Courtial and D. B. Dingwell, “Nonlinear composition dependence of molar volume of melts in the CaO-Al2O3-SiO2 system,” Geochimica et Cosmochimica Acta, vol. 59, pp. 3685-3695 (1995).
Q. Liu and R. A. Lange, “The partial molar volume and thermal expansivity of TiO2 in alkali silicate melts: Systematic variation with Ti coordination,” Geochimica et Cosmochimica Acta, vol. 65, pp. 2379-2393 (2001).
Y. Bottinga and D. F. Weill, “Densities of liquid silicate systems calculated from partial molar volumes of oxide components,” American Journal of Science, vol. 269, pp. 169-182 (1970).
Y. Bottinga, D. Weill, and P. Richet, “Density calculations for silicate liquids. I. Revised method for aluminosilicate compositions,” Geochimica et Cosmochimica Acta, vol. 46, pp. 909-919 (1982).
Y. Bottinga, P. Richet, and D. F. Weill, “Calculation of the density and thermal expansion coefficient of silicate liquids,” Bulletin de Mineralogie, vol. 106, pp. 129-138 (1983).
Acknowledgments
Financial support from Hyundai Steel, JFE Steel Corporation, Nippon Steel & Sumitomo Metal, Nucor Steel, Posco, RioTinto, RHI, RIST, Tata Steel Europe, Voestalpine, and the Natural Science and Engineering Research Council of Canada (NSERC) is gratefully acknowledged. One of the authors (E.T.) also acknowledges the scholarship from the Fonds de Recherche du Quebec - Nature et Technologies (FRQNT) supporting his master study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 27, 2014.
Rights and permissions
About this article
Cite this article
Thibodeau, E., Gheribi, A.E. & Jung, IH. A Structural Molar Volume Model for Oxide Melts Part III: Fe Oxide-Containing Melts. Metall Mater Trans B 47, 1187–1202 (2016). https://doi.org/10.1007/s11663-015-0549-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0549-x