Abstract
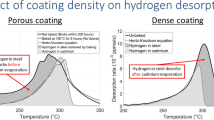
Thermal desorption spectroscopy (TDS) and sustained-load tests were used to investigate hydrogen embrittlement (HE), hydrogen profiles and the impact of the baking delay of cadmium-plated 4340 steel-notched bars. The results show a tremendous concentration of hydrogen in the cadmium coatings (200 to 1500 ppma) that does not contribute to HE. Hydrogen in manganese sulfide (MnS) traps was also found not to contribute to HE. The total hydrogen concentration measured by TDS is thus not suitable to determine an embrittlement threshold. Subtracting hydrogen in cadmium and MnS traps from total hydrogen allowed finding a true critical hydrogen concentration threshold of 0.6 ppma (0.01 ppmm). No impact of the baking delay was found.









Similar content being viewed by others
Notes
We take the hydrogen electrode as the 0 V potential reference.
In this paper, “ppma” means “ppm atomic” and “ppmm” means “ppm mass” (the conversion factor for hydrogen in iron is 1 ppmm \(= 56\) ppma).
When \(x(t) = 0\), all the hydrogen is still inside the sample. When \(x(t) = 1\), the hydrogen has completely desorbed (i.e. it has totally reacted), and the desorption rate at this point is 0 for the trap of activation energy \(E_{\text{a}}\).
[H]\(_{Steel}\) is the ratio between the number of hydrogen atoms measured divided by the number of iron atom in the steel sample. To get ppma, that number is multiplied by \(10^6\).
Note that this value is not affected by the error introduced by the background removal (for temperature between \(350\,{}^{\circ }{\text {C}}\) and \(500\,{}^{\circ }{\text {C}}\)), since the hydrogen in Figure 8(d) desorbs at temperatures below \(350\,{}^{\circ }{\text {C}}\).
References
W. H. Johnson, Proc. R Soc. Lond., 1874, vol. 23, pp. 168–179.
M. Wang, E. Akiyama, and K. Tsuzaki, Corr. Sci., 2006, vol. 48, pp. 2189–2202.
M. Wang, E. Akiyama, and K. Tsuzaki, Mater. Sci. Eng. A, 2005, vol. 398, pp. 37–46.
A. R. Troiano, Corrosion, 1959, vol. 15, pp. 57–62.
L. B. Pfeil, Proc. R Soc. Lond., 1926, vol. A112, pp. 128–195.
R. A. Oriani, Corrosion, 1987, vol. 43, pp. 390–397.
W. W. Gerberich, R. A. Oriani, M.-J. Lji, X. Chen, and T. Foecke, Philosoph. Mag. A, 1991, vol. 63, pp. 363–376.
D. E. Jiang and E. A. Carter, Acta Mater., 2004, vol. 52, pp. 4801–4807.
S.P. Lynch: NACE Int., 2007, pp. 1–55.
Z. Tarzimoghadam, M. Rohwerder, S.V. Merzlikin, A. Bashir, L. Yedra, S. Eswara, D. Ponge, and D. Raabe, Acta Mater., 2016, vol. 109, pp. 69–81.
H. K. Birnbaum and P. Sofronis, Mater. Sci. Eng., 1994, vol. 176, pp. 191– 202.
P. J. Ferreira, I. M. Robertson, and H. K. Birnbaum, Acta Mater., 1998, vol. 46, pp. 1749–1757.
I. M. Robertson, Eng. Fract. Mech., 2001, vol. 68, pp. 671–692.
I.M. Robertson, P. Sofronis, A. Nagao, M. L. Martin, S. Wang, D. W. Gross, and K. E. Nygren, Metall. Mater. Trans. A, 2015, vol. 46A, pp. 2323–2341.
S. P. Lynch, Acta Metall., 1988, vol. 20, pp. 2639–2661.
D. Figueroa and M. J. Robinson, Corr. Sci., 2010, vol. 52, pp. 1593–1602.
M. B. Djukic, Gordana M. B., V. S. Zeravcic, A. Sedmak, and B. Rajicic, Corrosion, 2016, vol. 72, pp. 943–961.
M. Devanathan, Z. Stachurski, and W. Beck, J. Electrochem. Soc., 1963, vol. 110, pp. 886–890.
D. A. Berman, Mater. Perform., 1985, vol. 24, pp. 36–41.
K R Sriraman, S Brahimi, J A Szpunar, and S Yue, J. Appl. Electrochem., 2013, vol. 43, pp. 441–451.
M. Zamanzadeh, A. Allam, C. Kato, B. Ateya, and H. W. Pickering, J. Electrochem. Soc., 1982, vol. 129, pp. 284–289.
K. Koenig, A. N. Lasseigne, J. W. Cisler, B. Mishra, R.H. King, and D.L. Olson, Int. J. Press. Vess. Pip., 2010, vol. 87, pp. 605–610.
S. M. Beloglazov, J. Alloys Compd., 2003, vol. 356-357, pp. 240–243.
D. R. Gabe, J. Appl. Electrochem., 1997, vol. 27, pp. 908–915.
T. J. Tuaweri, E. M. Adigio, and P. P. Jombo, Int. j. eng. sci. invention res. dev., 2013, vol. 2, pp. 17–24.
W. Blum and L. Farber, Bureau Stand. J. Res., 1930, vol. 4, pp. 27–39.
W. Beck, A. L. Glass, and E. Taylor, Aeronautical Materials Laboratory,, 1965, vol. 112, pp. 53–59.
A. E. Yaniv, T. P. Radhakrishnan, and L. L. Shreir, Transactions of the IMF, 1967, vol. 45, pp. 1–9.
A. Turnbull, R. B. Hutchings, and D. H. Ferriss, Mater. Sci. Eng. A, 1997, vol. 238, pp. 317–328.
H. E. Kissinger, Analytical Chemistry, 1957, vol. 29, pp. 1702–1706.
J. L. Lee and J.Y. Lee, Metal Science, 1983, vol. 17, pp. 426–432.
H. G. Lee and J.Y. Lee, Acta Metallurgica, 1984, vol. 32, pp. 131–136.
H. Bhadeshia, ISIJ International, 2016, vol. 56, pp. 24–36.
F. G. Wei, T. Hara, and K. Tsuzaki, Metallurgical and Materials Transactions B, 2004, vol. 35, pp. 587–597.
H. J. Grabke and E. Riecke, Materials Technology, 2000, vol. 34, pp. 331–342.
W. Blum, W.P. Barrows, and A. Brenner, Bureau of Standards Journal of Research, 1931, vol. 7, p. 697.
Acknowledgements
This work was supported by the Consortium de Recherche et d’Innovation en Aérospatiale au Québec (CRIAQ), the Natural Sciences and Engineering Research Council of Canada (NSERC), as well as the Fonds de Recherche du Québec—Nature et technologies (FRQNT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted April 29, 2019.
Rights and permissions
About this article
Cite this article
Bellemare, J., Laliberté-Riverin, S., Ménard, D. et al. Subtleties Behind Hydrogen Embrittlement of Cadmium-Plated 4340 Steel Revealed by Thermal Desorption Spectroscopy and Sustained-Load Tests. Metall Mater Trans A 51, 3054–3065 (2020). https://doi.org/10.1007/s11661-020-05741-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-020-05741-0