Abstract
Objective
To assess the modulatory impact of alcoholic extract of fruit of Mengkudu (AEFM, Morinda citrifolia L., Rubiaceae) on renal oxido-lipidemic stress in hypercholesterolemic albino rats.
Methods
Twenty-four male albino rats were randomly divided into four groups with six rats in each group: group I as control, group II fed with hypercholesterolemic diet (HCD) for 45 days (4% cholesterol and 1% cholic acid), Group III rats fed with HCD for 45 days + AEFM (300 mg/kg body weight/day orally) for last 30 days and group IV normal rats fed AEFM alone. The blood was collected using ethylenediamine tetraacetic acid (EDTA) as an anticoagulant for various biochemical analysis, and excision of kidney was done for histological analysis.
Results
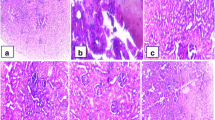
The levels of total cholesterol (TC), triacylglycerol (TG), phospholipids (PLs), renal functional parameters and lipid peroxidation products were markedly mitigated in AEFM treated hypercholesterolemic rats (group III) compared to group I (P<0.01). Activities of both enzymic and non-enzymic free radical scavenging factors were significantly increased in group III compared to group I (P<0.01). In group III the mRNA levels of interstitial endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) genes were obviously up-regulated (P<0.01) and down-regulated in (P<0.05) compared with group I. Histomorphological observations also exhibited similar as in group III AEFM commendably protects the renal tissues compared with group I (P<0.01).
Conclusion
AEFM can act as nephroprotective agent by attenuating the renal oxidative stress, lipid levels as well as regulating NOS level and by this means protects the kidney in hypercholesterolemic induced nephropathy experimental rats.
Similar content being viewed by others
References
Arora MK, Reddy K, Balakumar P. The low dose combination of fenofibrate and rosiglitazone halts the progression of diabetes-induced experimental nephropathy. Euro J Pharmacol 2010;636:137–144.
Chade AR, Krier JD, Galili O, Lerman A, Lerman LO. Role of renal cortical neovascularization in experimental hypercholesterolemia. Hypertension 2007;50:729–736.
Montilla P, Espejo I, Munoz MC, Bujalance I, Munoz-Castaneda JR, Tunez I. Protective effect of red wine on oxidative stress and antioxidant enzyme activities in the brain and kidney induced by feeding high cholesterol in rats. Clin Nut 2006;25:146–153.
Deji N, Kume S, Araki S, Soumura M, Sugimoto T, Isshiki K, et al. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol Renal Physiol 2009;296:F118–F126.
Feldstein A, Krier JD, Sarafov MH. In vivo renal vascular and tubular function in experimental hypercholesterolemia. Hypertension 1999;34:859–864.
Yao Y, Tian XK, Liu XC, Shao JF. Early renal morphological changes in high cholesterol diet rats model. J Nat Sci 2005;10:1063–1068.
Parthasarathy S, Santanam N, Ramachandran S, Meilhac O. Potential role of oxidized lipids and lipoproteins in antioxidant defense. Free Radic Res 2000;33:197–215.
McClatchey W. From Polynesian healers to health food stores: changing perspectives of morinda citrifolia (Rubiaceae). Integr Cancer Ther 2002;1:110–120.
Nelson, SC. Noni cultivation in Hawaii. Fruit and Nuts 2001;4:1–4
Tabrah FL, Eveleth BM. Evalution of the effectiveness of ancient Hawaiian medicine. Hawaii Med J 1966;25:223–230.
Morton JF. The ocean-going Noni, or Indian mulberry (Morinda citrifolia, Rubiaceae) and some of its ‘colorful’ relatives. Ecological Botony 1992;46:241–256.
Kahiolo GW, Mookini T, Neizmen EC, Tom D, The Story of Kamapuaa. Manoa, Honolulu, Hawaii: Hawaiian Studies Program, University of Hawaii; 1978.
Lin YL, Chou CH, Yang DJ, Chen JW, Tzang BS, Chen YC. Hypolipidemic and antioxidative effects of Noni (Morinda citrifolia L.) juice on high- fat/cholesterol-dietary hamsters. Plant Foods for Human Nutrition 2012;67:294–302.
Mandukhail SR, Aziz1 N, Gilani AH. Studies on antidyslipidemic effects of Morinda citrifolia (Noni) fruit, leaves and root extracts. Lipids Health Dis 2010;9:88.
Folch J, Lees M, Sloane-Stanley GH. A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 1957;226:497–509.
Parekh AC, Jung DH. Cholesterol determination with ferric chloride–uranium acetate and sulfuric acid–ferrous sulfate reagents. Anal Chem 1970;42:1423–1427.
Rice EW. Triglycerides (neutral fat) in serum. Standard methods clinical chemistry. New York: Academic Press 1970:215–219
Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970;5:494–496.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974;47:469–474.
Sinha AK. Colorimetric assay of catalase. Anal Biochem, 1972;47:389–394.
Rotruck JT, Pope AL, Ganther HE. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973;179:588–590.
Stall GEJ, Visser J, Veeger C. Purification and properties of glutathione reductase of human erythrocytes. Biochim Biophys Acta 1969;185:39–48.
Habig WH, Pabst MJ, Jakoby WB. Glutathione-Stransferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–7139.
Moron MS, Depierre JW, Mannervik B. Levels of glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 1979;582:67–78.
Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol 1979;62:3–11.
Baker AF, Frank G. Estimation of Vitamin E in tissues. In: Bollinger G, ed. Dunnshchicht, chromatographic in laboratorium "Hand brich". Berlin: Springer-Verlag 1951;41–52.
Devasagayam TPA, Tarachand U. Decreased lipid peroxidation in rat kidney during gestation. Biochem Biophys Res Commun 1987;145:134–138.
Lowry OH, Rosebrough NJ, Farr AL, Randall R. Protein measurement with Folin’s phenol reagent. J Biol Chem 1951;193:265–275.
Saini HK, Arneja AS, Dhalla NS. Role of cholesterol in cardiovascular dysfunction. Can J Cardiol 2004;20:333–336.
Scheuer H, Gwinner W, Hohbach J, Gröne EF, Brandes RP, Malle E, et al. Oxidant stress in hyperlipidemia-induced renal damage. Am J Physiol Renal Physiol 2000;278:63–74.
Grone EF, Walli AK, Grone HJ, Miller B, Seidel D. The role of lipids in nephrosclerosis and glomerulosclerosis. Atherosclerosis 1994;107:1–13.
Al-Rejaie SS, Abuohashish HM, Alkhamees OA, Aleisa AM, Alroujayee AS. Gender difference following high cholesterol diet induced renal injury and the protective role of rutin and ascorbic acid combination in Wistar albino rats. Lipids Health Dis 2012;11:41–50.
Sorimuthu Subramanian, US Mahadeva Rao. Amelioration of diabetic dyslipidemia by Morinda citrifolia fruits on streptozotocin induced diabetic rats. Pharm Res 2010;3:843-848.
Salem NA, Salem EA. Renoprotective effect of grape seed extract against oxidative stress induced by gentamicin and hypercholesterolemia in rats. Ren Failure 2011;33:824–832.
Akpolat M, Kanter M, Topcu-Tarladacalisir Y, Aydogdu N. Protective effect of flaxseed oil on renal injury in hyperlipidaemic rats, the effect of flaxseed oil on hyperlipidaemia. Phytother Res 2011;25:796–802.
Ishiyama A, Atarashi K, Minami M, Takagi M, Kimura K, Goto A, et al. Role of free radicals in the pathogenesis of lipid-induced glomerulosclerosis in rats. Kidney Int 1999;55:1348–1358.
Green CO, Wheatley AB, McGrowder DA, Dilworth LL, Asemota HN. Modulation of antioxidant enzymes activities and lipid peroxidation products in diet-induced hypercholesterolemic rats fed ortanique peel polymethoxylated flavones extract. J App Biomed 2012;10:91–101.
Deepa PR, Varalakshmi P. Salubrious effect of low molecular weight heparin on atherogenic diet-induced cardiac, hepatic and renal lipid peroxidation and collapse of antioxidant defences. Mol Cell Biochem 2003;254:111–116.
Mahadeva US R, Subramanian S. Biochemical evaluation of antihyperglycemic and antioxidative effects of Morinda citrifolia fruit extract studied in streptozotocin-induced diabetic rats. Med Chem Res 2009;18:433–446.
Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Oxidtive DNA damage and repair in experimental atherosclerosis are reversed by dietary lipid lowering. Cir Res 2001;88:733–739.
Deepa PR, Varalakshmi P. Atheroprotective effect of endogenous heparin-derivative treatment on the aortic distrubances and lipoprotein oxidation in hypercholesterolemic diet fed rats. Clin Chim Acta 2005;355:119–130.
Chenni A, Yahia DA, Boukortt FO, Prost J, Lacaille-Dubois MA, Bouchenak M. Effect of aqueous extract of Ajuga iva supplementation on plasma lipid profile and tissue antioxidant status in rats fed a high-cholesterol diet. J Ethnopharmacol 2007;109:207–213.
Feldstein A, Krier JD, Sarafou MH, Lerman A, Best PJ, Wilson SH, et al. In vivo renal vascular and tubular function in experimental hypercholesterolemia. Hypertension 1999;34:859–864.
Scheuer H1, Gwinner W, Hohbach J, Gröne EF, Brandes RP, Malle E, et al. Oxidant stress in hyperlipidemiainduced renal damage. Am J Physiol Renal Physiol 2000;278:F63–F74.
Yadav YC, Srivastava DN. Nephroprotective and curative effects of Ficus religiosa latex extract against cisplatin-induced acute renal failure. Pharmaceutical Biol 2013;51:1480–1485.
Akpolat M, Kanter M, Topcu-Tarladacalisir Y, Aydogdu N. Protective effect of flaxseed oil on renal injury in hyperlipidaemic rats: the effect of flaxseed oil on hyperlipidaemia. Phytother Res 2011;25:796–802.
Stulak JM, Lerman A, Caccitolo JA, Wilson SH, Romero JC, Schaff HV, et al. Impaired renal vascular endothelial function in vitro in experimental hypercholesterolemia. Atherosclerosis 2001;154:195–201.
Plotnikov EY, Chupyrkina AA, Pevzner IB, Isaev NK, Zorov DB. Myoglobin causes oxidative stress, increase of NO production and dysfunction of kidney’s mitochondria. Biochim Biophys Acta 2009;1792:796–803.
Sandovici M, Henning RH, Hut RA, Strijkstra AM, Epema AH, van Goor H, et al. Differential regulation of glomerular and interstitial endothelial nitric oxide synthase expression in the kidney of hibernating ground squirrel. Nitric Oxi 2004;11:194–200.
Tain YL, Muller V, Szabo AJ, Erdely A, Smith C, Baylis C. Renal cortex neuronal nitric oxide synthase in response to rapamycin in kidney transplantation. Nitric Oxi 2008;18:80–86.
Bhatia S, Shukla R, Venkata MS, Kaur GJ, Madhava PK. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin Biochem 2003;36:557–562.
Zhou XJ, Laszik Z, Wang XQ, Silva FG, Vaziri ND. Association of renal injury with increased oxygen free radical activity and altered nitric oxide metabolism in chronic experimental hemosiderosis. Lab Invest 2000;80:1905–1914.
Amin KA, Kamel HH, Abd Eltawab MA. Protective effect of garcinia against renal oxidative stress and biomarkers induced by high fat and sucrose diet. Lipids Health Dis 2011;10:6–19.
Li H, Forstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr Opinion Pharmacol 2013;13:161–167.
Wessells H, Teal TH, Luttrell IP, Sullivan CJ. Effect of endothelial cell-based iNOS gene transfer on cavernosal eNOS expression and mouse erectile responses. Int J Imp Res 2006;18:438–445.
Ricardo SD, Van Goor H, Diamond JR. Hypercholesterolemia and progressive kidney disease, the role of macrophages and macrophage-derived products. Contrib Nephrol 1997;120:197–209.
Ishiyama A, Atarashi K, Minami M, Takagi M, Kimura K, Goto A, et al. Role of free radicals in the pathogenesis of lipid-induced glomerulosclerosis in rats. Kidney Int 1999;55:1348–1358.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interests The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Mahadeva Rao, U.S., Shanmuga Sundaram, C. Antihypercholesterolemic, antioxidant and renal protective effects of Mengkudu (Rubiaceae) fruit in nephropathy-induced albino rats. Chin. J. Integr. Med. (2017). https://doi.org/10.1007/s11655-017-2785-1
Received:
Published:
DOI: https://doi.org/10.1007/s11655-017-2785-1