Abstract
Background
The objective of present study was to provide the pharmacological basis for the medicinal use of Morinda citrifolia Linn in dyslipidemia using the aqueous-ethanolic extracts of its fruits (Mc.Cr.F), leaves (Mc.Cr.L) and roots (Mc.Cr.R).
Results
Mc.Cr.F, Mc.Cr.L and Mc.Cr.R showed antidyslipidemic effects in both triton (WR-1339) and high fat diet-induced dyslipidemic rat models to variable extents. All three extracts caused reduction in total cholesterol and triglyceride levels in triton-induced dyslipidemia. In high fat diet-induced dyslipidemia all these extracts caused significant reduction in total cholesterol, triglyceride, low density lipoprotein-cholesterol (LDL-C), atherogenic index and TC/HDL ratio. Mc.Cr.R extract also caused increase in high density lipoprotein-cholesterol (HDL-C). The Mc.Cr.L and Mc.Cr.R reduced gain in body weight with a reduction in daily diet consumption but Mc.Cr.F had no effect on body weight and daily diet consumption.
Conclusions
These data indicate that the antidyslipidemic effect of the plant extracts was meditated through the inhibition of biosynthesis, absorption and secretion of lipids. This may be possibly due partly to the presence of antioxidant constituents in this plant. Therefore, this study rationalizes the medicinal use of Morinda citrifolia in dyslipidemia.
Similar content being viewed by others
Background
Dyslipidemia is an independent and modifiable risk factor for cardiovascular diseases. Its prevalence is growing not only in developed countries but also in developing countries [1]. Treatment of dyslipidemia reduces cardiovascular events [2]. The modern pharmacological therapy for abnormal lipids is effective but is costly and associated with side-effects [3] leading to patient incompliance. Therefore, alternative therapies particularly, herbal based are being explored. Morinda citrifolia Linn (Fam. Rubiaceae) is commonly known as Noni. Different parts of the plant including fruit, leaves, root, stem and bark are used in folk medicine in Polynesia, Tahiti, Southeast Asia, Australia and Hawaii. It has been shown that these are effective against minimizing the symptoms of life style-related diseases such as atherosclerosis [4], hypertension [5] and other vascular disorders [4], stroke [6], diabetes and cancer [7]. Furthermore, Noni juice, a popular beverage is known to contain some antioxidative and anti-inflammatory ingredients [5].
Morinda citrifolia, has been reported to possess vasodilatory [8, 9], antioxidant [10] antitumor [11] and Angiotensin Converting Enzyme inhibitor activities[12]. It is an edible plant and its fruit juice is a popular drink. Almost all parts of this plant have some medicinal value and have been widely studied phytochemically [see additional file 1].
Recently we have reported that antispasmodic and vasodilatory activities of Morinda citrifolia root extract are mediated through blockade of voltage-dependent calcium channels [9]. However, the plant is not widely studied for its antidyslipidemic effects except a preliminary report [13]. Therefore, the objective of the present study was to investigate the antidyslipidemic effect of Morinda citrifolia in Triton WR 1339 and high fat diet-induced dyslipidemia rat models to rationalize its medicinal use in cardiovascular disorders.
Materials and methods
Plant material
The vacuum dried 70% aqueous-ethanolic extract of Morinda citrifolia fruit, leaves and roots were gifted by the Sami Labs Limited 19/1, 19/2, 1st Main II Phase, Peenya Industrial Area, Bangalor-560 058, India. The extracts were prepared by following procedure as described by the manufacturer. Dried leaves, fruit and roots of Morinda citrifolia were procured from a reputed herb supplier in Southern India. The leaves, fruits and roots were chopped and ground by hammer mill and passed through 20 mesh screen. The powder (12 Kg) was extracted with 70% ethanol (48 L) at 70°C for 3 hours and filtered. The procedure repeated twice and all filtrates combined and evaporated under vacuum. The dried extracts were packed in polythene bags with nitrogen purge.
Drugs and standards
The following reference chemicals were obtained from the source specified: Triton (tyloxapol), Cholic acid, cholesterol (Sigma Chemical Company, ST Louis, MO, USA) and diethylether (Sigma-Aldrich Chemie GmbH, Germany). Commercial Randox diagnostic kits were used for serum analyses of total cholesterol (TC), triglyceride (TG), high density lipoprotein-cholesterol (HDL-C) and glucose level (Randox Laboratories Ltd., Co. Antrim, UK). All chemicals used were of the highest purity grade.
Animals
Sprague-Dawley (SD) rats (180-220 g) and mice (20-25 g) of either sex were obtained from the animal house of the Aga Khan University, Karachi. The animals were housed in constant room temperature (23-25°C), kept in plastic cages (47 × 34 × 18 cm3) with sawdust (renewed after every 48 h) and had free access to food and water. Rats were starved for 16 hrs prior to experiment, anaesthetized with diethyl ether by inhalation and blood was collected via cardiac puncture. Experiments performed with the rulings of the Institute of Laboratory Animal Resources, Commission on Life Sciences [14].
Preparation of diet
The following two types of diets were prepared.
-
1.
Normal diet: The normal diet was prepared at the animal house of the Aga Khan University (AKU), Karachi. The standard diet consists of flour (5 kg), chokar (5 kg), salt (75 g), nutrivet L (33 g), molasses (150 g), potassium meta bisulphate (15 g), fish meal (2.25 kg), powdered milk (2 kg) and oil (500 g) for a total of about 15 kg of the food material.
-
2.
Atherogenic diet: Cholesterol (2% w/w), cholic acid (0.5% w/w) and butter fat (5% w/w) were added to normal diet, as described by Ichihashi [15] with slight modification.
All measures were taken to ensure the uniform mixing of additives in dry ingredients of the diet before kneading.
Triton WR 1339-induced model of hyperlipidemia
The tyloxapol-induced hyperlipidemic model earlier described by Khanna [16] was followed with slight modifications. Male SD rats weighing 180-220 g caged in uniform hygienic conditions and kept on standard pellet diet and water ad libitum. The rats were randomly divided into 8 groups, each containing 6 rats, 1) control group, 2) tritonized group (untreated) and 3-8) treated groups with plant materials (Mc.Cr.F, Mc.Cr.R and Mc.Cr.L). After 10 days of the treatment, all animals were fasted for 7 h and group 1 received saline (10 ml/kg; i.p.), while groups 2-8 were given tyloxapol (500 mg/kg; i.p.). On the next day, rats were anaesthetized with diethylether by inhalation and blood was collected via cardiac puncture for analysis of serum total cholesterol and triglycerides.
High fat diet-induced model
The high fat diet-induced dyslipidemic model was used as earlier described [17] with slight modifications after pilot studies. The adult SD rats (180-220 g) were randomly divided into different groups, each containing 6 rats. Control group was given normal diet (served as normal control), untreated group given atherogenic diet (atherogenic control) and treated groups were given atherogenic diet plus drug administered orally. All the groups of animals had free access to water and diet. The diet consumption was monitored daily and the gain in body weight was monitored weekly. At the end of the treatment, rats were fasted for 16 hrs, anaesthetized with diethyl ether by inhalation, blood was collected via cardiac puncture and serum analyzed for lipid profile and glucose level.
Biochemical studies
Estimation of lipid profile and glucose level
For the determination of serum total cholesterol, high density lipoprotein cholesterol, triglyceride and glucose, a methods described by the manufacture (Randox Laboratories Ltd., Co. Antrim, UK.) was used. For this method test sample (serum), standard and blank were pipetted using a micropipette in to eppendorf tube. The reaction mixtures were mixed well and incubated at 20- 25°C. After incubation, 0.25 ml of each reaction mixture was poured in 96 well plates and the absorbance was read at 490 nm against the reagent blank in micro plate reader (Model 680 Bio-Rad Laboratories UK Ltd). The concentration of absorbance was calculated from the slope of concentration curve of the standards.
Estimation of LDL-C, TC/HDL and atherogenic index
These result were calculated indirectly by using formula describe by [18].
LDL = TC - HDL - TG/5 and Atherogenic index= TC-HDL/HDL.
Acute toxicity
The test was performed as described earlier [18]. Animals were divided in different groups of 5 mice each and were administered increasing doses of the plant extracts (3, 5, 10 g/kg, p.o.), in 10 ml/kg volume. Another group of mice was administered saline (10 ml/kg, p.o.) served as a negative control. The mice were allowed food and water ad libitum during a 24 hr test period and kept under regular observation for gross behavioural changes and mortality.
Data analysis
All data were expressed as mean ± standard error of mean (SEM, n = number of experiments) and EC50 values are given as geometric mean with 95% confidence intervals (CI). One-way Analysis of Variance (one-way ANOVA) was used to compare the differences in means of more than two groups, followed by Tukey post-test to determine significant differences among the pairs. P-values less than 0.05 (p < 0.05) were considered as statistically significant. All the graphs, calculation and statistical analyses were performed using GraphPad Prism software version 4.00 for Windows (GraphPad Software, San Diego California USA, http://www.graphpad.com).
Results
Effect of Morinda citrifolia extracts on tyloxapol-induced hyperlipidemia
Administration of tyloxapol (triton WR-1339) caused a significant increase (p < 0.001) in serum total cholesterol and triglyceride of tritonized group as compared to animals in the control group. Pretreatment of the rats with Mc.Cr.F (1000 mg/kg), Mc.Cr.L (1000 mg/kg) and Mc.Cr.R (500 mg/kg) caused significant reduction in cholesterol and triglyceride level. The data are summarized in Table 1.
Effect of Fruit extract on high fat diet-induced dyslipidemia
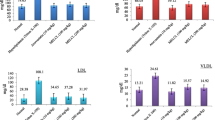
The intake of atherogenic diet increased serum total cholesterol (TC), triglyceride, LDL-C, TC/HDL ratio, atherogenic index and glucose level as compared to the control group. The oral administration of fruit extract (1000 mg/kg) with atherogenic diet prevented the rise in serum TC, LDL-C, TC/HDL ratio and atherogenic index. The treatment with the fruit extract had no significant effect (p > 0.05) on the HDL-C and glucose levels as compared to the atherogenic group. However, there was no effect seen on body weight and daily diet consumption (p > 0.05). The data are summarized in Table 2.
Effect of leaves extract on high fat diet-induced dyslipidemia
The TC, triglyceride, LDL-C, TC/HDL ratio, atherogenic index and glucose levels of the atherogenic group were significantly increased as compared to the control group. Oral administration of leaves extract (1000 mg/kg) with atherogenic diet prevented the rise of serum TC, LDL-C, TC/HDL ratio, atherogenic index and glucose levels. The HDL-C was similar (p > 0.05) to that in the atherogenic group. The leaves extract significantly prevented the gain in average body weight and increased daily diet consumption as compared to the atherogenic group. The data are summarized in Table 3.
Effect of root extract on high fat diet-induced dyslipidemia
The TC, triglyceride, LDL-C, TC/HDL ratio, atherogenic index and glucose levels in the atherogenic group were significantly increased as compared to the control group. Oral administration of root extract (500 mg/kg) with atherogenic diet prevented the rise in serum TC, LDL-C, TC/HDL ratio, atherogenic index and glucose levels. The treatment with (500 mg/kg) also increased HDL-C (p < 0.05) as compared to the atherogenic group. The root extract significantly prevented the gain in average body weight and increased daily diet consumption as compared to the atherogenic group (p < 0.001). The data are summarized in Table 4.
Safety study
The treatment with Morinda citrifolia fruit, leaves and root extracts for 10 day (tyloxapol study) and 6 weeks (high fat diet study) did not produce any death or behavioural changes in rats.
Acute toxicity testing (24 hour time) of Mc.Cr.F and Mc.Cr.L was conducted in mice at different doses (3, 5 and 10 g/kg) and there was no mortality or changes in gross behaviour observed up to the dose of as high as 10 g/Kg, as compared to control group. The roots extract (Mc.Cr.R) also did not cause any mortality and changes in gross behaviour up to the dose of 10 g/Kg as previously described [9].
Discussion
Morinda citrifolia has been considered useful in cardiovascular diseases particularly hypertension, atherosclerosis and dyslipidemia. In this study we used different animal models to evaluate the possible mode of action(s) of antidyslipidemic effect of different parts of Morinda citrifolia. Tyloxapol is a non-ionic surfactant being widely used to explore possible mechanism of lipid lowering drugs, it causes drastic increase in serum triglycerides and cholesterol levels due to increase in hepatic cholesterol synthesis particularly by the increase in HMG Co-A (3-hydroxy-3-methyl-glutaryl Co-A) activity [19] and by the inhibition of lipoprotein lipase responsible for hydrolysis of plasma lipids [20]. In fasting condition the only source of serum lipid levels is the endogenous production. Significant inhibition of rise in lipid levels by extracts of various parts of Morinda citrifolia in this model is indicative of inhibition of cholesterol biosynthesis by inhibition of HMG Co-A. This enzyme plays a key role in controlling lipid levels in plasma and other tissue. Most of the newer antidyslipidemic drugs such as statins act via the inhibition of HMG Co-A. However, failure of the Noni extracts to cause complete inhibition indicates the involvement of additional mechanisms. Morinda citrifolia is reported to be rich in flavones [9, 21, 22], which are known to inhibit lipid biosynthesis [23]. High cholesterol diet induces endothelial dysfunction, atherosclerosis [24] and increases oxidative stress by increasing the expression of oxidation-sensitive genes, such as Elk-1 and p-CREB [25]. High cholesterol diet with cholic acid increases TC, LDL-C, atherogenic index and decrease HDL-C by enhancing intestinal absorption and secretion and decreasing catabolism of cholesterol [26]. Treatment with Morinda citrifolia extracts caused a significant decrease in mean serum TC and LDL-C while increased HDL-C. The plant extracts also caused significant reduction in the atherogenic index, which is considered a better indicator of coronary heart disease risk than individual lipoprotein concentration [27].
High fat diet also causes oxidative stress (enzymatic and non-enzymatic) in rats, thus, increases oxidation of low density lipoprotein (LDL) which plays key role in genesis of atherosclerosis. Antioxidants are known to effectively prevent this kind of damage [28]. The presence of strong antioxidant activities in various parts of Morinda citrifolia [29] may offer additional benefit in combating the oxidative stress caused by high cholesterol. Relatively strong antidyslipidemic activity in root extract may be due to the presence of more antioxidant activity in this part [10, 29]. The active constituents responsible for antioxidant activity include 3,3'-bisdemethylpinoresinol, americanol A, morindolin, isoprincepin [30], scopoletin [31], kaempferol, ursolic acid, quercetin and various other constituents [30, 32–34] [see additional file 1]
Conclusions
The present study provides mechanisms of antidyslipidemic activities of various parts of Morinda citrifolia through multiple pathways i.e., inhibition of biosynthesis, absorption and secretion of lipids. This may be due to the presence of multiple potent antioxidant constituents in this plant, though additional mechanism(s) cannot be ruled out. The results from this study rationalize the medicinal use of Morinda citrifolia in dyslipidemia and it can be used as a potential medicine for cardiovascular diseases. However, further studies are required to prove the safety and efficacy of Morinda citrifolia and its constituents in actual clinical settings.
Abbreviations
- (MC.CR.F):
-
Morinda citrifolia fruit extract
- (MC.CR.L):
-
Morinda citrifolia leaves extract
- (MC.CR.R.):
-
Morinda citrifolia root extract
- (TC):
-
total cholesterol
- (TG):
-
triglyceride
- (LDL-C):
-
low density lipoprotein-cholesterol
- (HDL-C):
-
high density lipoprotein-cholesterol
- (SD):
-
Sprague-Dawley.
References
Paccaud F, Fasmeye VS, Wietlisbach V, Bovet P: Dyslipidemia and abdominal obesity: an assessment in three general populations. J Clin Epidemiol. 2000, 53 (4): 393-400. 10.1016/S0895-4356(99)00184-5
Ballantyne CM: Treatment of Dyslipidemia to Reduce Cardiovascular Risk in Patients with Multiple Risk Factors. Clin Cornerstone. 2007, 8 (6): S6-S13. 10.1016/S1098-3597(07)80010-X
Grundy SM, Cleeman JI, Merz CN, Brewer HB, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Stone NJ: National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004, 110 (2): 227-239. 10.1161/01.CIR.0000133317.49796.0E
Wang MY, West BJ, Jensen CJ, Nowicki D, Chen SU, Palu AK, Anderson G: Morinda citrifolia (Noni) A literature review and recent advances in Noni research. Acta Pharmacologica Sinica. 2002, 23 (12): 1127-1141.
Harada S, Hamabe W, Kamiaya K, Satake T, Yamamoto J, Tokuyama S: Preventive Effect of Morinda citrifolia Fruit Juice on Neuronal Damage Induced by Focal Ischemia. Biol Pharm Bull. 2009, 32 (3): 405-409. 10.1248/bpb.32.405
Nayak BS, Sandiford S, Maxwell A: Evaluation of the Wound-healing Activity of Ethanolic Extract of Morinda citrifolia L. Leaf. Evid Based Complement Alternat Med. 2009, 6 (3): 351-356. 10.1093/ecam/nem127
Takashima J, Ikeda Y, Komiyama K, Hayashi M, Kishida A, Ohsaki A: New Constituents from the Leaves of Morinda citrifolia. Chem Pharm Bull. 2007, 55 (2): 343-345. 10.1248/cpb.55.343
Runnie I, Salleh MN, Mohamed S, Head RJ, Abeywardena MY: Vasorelaxation induced by common edible tropical plant extracts in isolated rat aorta and mesenteric vascular bed. J Ethnopharmacol. 2004, 92 (2-3): 311-316. 10.1016/j.jep.2004.03.019
Gilani AH, Mandukhail SR, Iqbal J, Yasinzai M, Aziz N, Khan A, Rehman N: Antispasmodic and vasodilator activities of Morinda citrifolia r oot extract are mediated through blockade of voltage dependent calcium channels. BMC Complement Altern Med. 2010, 10: 2- 10.1186/1472-6882-10-2
Zin ZM, Hamid AA, Osman A: Antioxidative activity of extracts from Mengkudu (Morinda citrifolia L.) root, fruit and leaf. Food Chem. 2002, 78: 227-231. 10.1016/S0308-8146(01)00402-2.
Furusawa E, Hirazumi A, Story S, Jensen J: Antitumour potential of a polysaccharide-rich substance from the fruit juice of Morinda citrifolia (Noni) on sarcoma 180 ascites tumour in mice. Phytother Res. 2003, 17 (10): 1158-64. 10.1002/ptr.1307
Shinya Y, Juno O, Masanobu S, Isafumi M, Yasuhiro O, Yoji T: Inhibition of angiotensin I converting enzyme by Noni [Morinda citrifolia] juice. Journal of the Japanese Society for Food Science and Technology. 2002, 49 (9): 624-627.
Nolting J, Cheerva A, Jensen J, Anderson G, Nowicki D, Story S: The effects of Morinda citrifolia (Noni) fruit juice on serum cholesterol and triglyceride in current smokers. Circulation. 2006, 113: 301-381.
National Research Council: Guide for the Care and Use of Laboratory Animals. 1996, Washington: National Academy Press, 1996
Ichihashi T, Izawa M, Miyata K, Mizui T, Hirano K, Takagishi Y: Mechanism of hypocholesterolemic action of S-8921 in rats: S-8921 inhibits ileal bile acid absorption. J Pharmacol Exp Ther. 1998, 284 (1): 43-50.
Khanna AK, Rizvi F, Chander R: Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J Ethnopharmacol. 2002, 82 (1): 19-22. 10.1016/S0378-8741(02)00136-8
Berroughui H, Ettaib A, Gonzalez H, Alvarez de Sotomayor M, Bennari- Kabchi N, Hmamouchi M: Hypolipidemic and hypocholesterolemic effect of argan oil (Argania spinosa L.) in Meriones Shawi rats. J Ethnopharmacol. 2003, 89 (1): 15-18. 10.1016/S0378-8741(03)00176-4
Aziz N, Mehmood MH, Mandukhail SR, Bashir S, Raoof S, Gilani AH: Antihypertensive, antioxidant, antidyslipidemic and endothelial modulating activities of a polyherbal formulation (POL-10). Vascul Pharmacol. 2009, 50 (1-2): 57-64. 10.1016/j.vph.2008.09.003
Kuroda M, Tanzawa K, Tsujita Y, Endo A: Mechanism for elevation of hepatic cholesterol synthesis and serum cholesterol levels in Triton WR-1339-induced hyperlipidemia. Biochim Biophys Acta. 489 (1): 119-125.
Schotz MC, Scanu A, Page IH: Effect of Triton on lipoprotein lipase of rat plasma. Am J Physiol. 1957, 188 (2): 399-402.
Nandhasri P, Pawa KK, Kaewtubtim J, Jeamchanya C, Jansom C, Sattaponpun C: Nutraceutical properties of Thai "Yor" Morinda citrifolia and "Noni" juice extract. Songklanakarin J Sci Technol. 2005, 27 (2): 579-586.
Ramamoorthy PK, Bono A: Antioxidant activity, total phenolic and flavonoid content of Morinda citrifolia fruit extracts from various extraction processes. J Engineering Sci Technol. 2007, 2 (1): 70-80.
Glasser G, Graefe EU, Struck F, Veit M, Gebhardt R: Comparison of antioxidative capacities and inhibitory effects on cholesterol biosynthesis of quercetin and potential metabolites. Phytomedicine. 2002, 9 (1): 33-40. 10.1078/0944-7113-00080
Hayashi T, Ishikawa T, Naito M, Kuzuya M, Funaki C, Asai K, Kuzuya F: Low level hyperlipidemia impairs endothelium-dependent relaxation of porcine coronary arteries by two mechanisms. Functional change in endothelium and impairment of endothelium-dependent relaxation by two mediators. Atherosclerosis. 1991, 87 (1): 23-38. 10.1016/0021-9150(91)90229-V
de Nigris F, Lerman A, Ignarro LJ, Williams-Ignarro S, Sica V, Baker AH, Lerman LO, Geng YJ, Napoli C: Oxidation-sensitive mechanisms, vascular apoptosis and atherosclerosis. Trends Mol Med. 2003, 9 (8): 351-359. 10.1016/S1471-4914(03)00139-4
Heuman DM, Vlahcevic ZR, Bailey ML, Hylemon PB: Regulation of bile acid synthesis. II. Effect of bile acid feeding on enzymes regulating hepatic cholesterol and bile acid synthesis in the rat. Hepatology. 1988, 8 (4): 892-897. 10.1002/hep.1840080431
Vijayakumar RS, Surya D, Nalini N: Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Rep. 2004, 9 (2): 105-110. 10.1179/135100004225004742
Kinosian B, Glick H, Garland G: Cholesterol and Coronary Heart Disease: Predicting Risks by Levels and Ratios. J investig Med. 1995, 43 (5): 443-450.
Krishnaiah D, Sarbatly R, Neh NL: Recovery of phytochemical components from various part of Morinda citrifolia extracts by using member separator. J Appl Sci. 2007, 7 (15): 2093-2098. 10.3923/jas.2007.2093.2098. 10.3923/jas.2007.2093.2098
Kamiya K, Tanaka Y, Endang H, Umar M, Satake T: Chemical constituents of Morinda citrifolia fruits inhibit copper-induced low-density lipoprotein oxidation. J Agric Food Chem. 2004, 22;52 (19): 5843-8. 10.1021/jf040114k. 10.1021/jf040114k
Shaw CY, Chen CH, Hsu CC, Chen CC, Tsai YC: Antioxidant properties of scopoletin isolated from Sinomonium acutum. Phytother Res. 2003, 17 (7): 823-5. 10.1002/ptr.1170
Takashima J, Ikeda Y, Komiyama K, Hayashi M, Kishida A, Ohsaki A: New constituents from the leaves of Morinda citrifolia. Chem Pharm Bull (Tokyo). 2007, 55 (2): 343-5. 10.1248/cpb.55.343
Kamiya K, Hamabe W, Harada S, Murakami R, Tokuyama S, Satake T: Chemical constituents of Morinda citrifolia roots exhibit hypoglycemic effects in streptozotocin-induced diabetic mice. Biol Pharm Bull. 2008, 31 (5): 935-8. 10.1248/bpb.31.935
Sattar FA: Studies On The Chemical Constituents Of Various Parts Of Morinda Citrifolia Linn.(Noni). PhD thesis. H.E.J. Research Institute of Chemistry/University of Karachi. 1995, http://eprints.hec.gov.pk/2274/1/2129.htm
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SRM carried out the experimental work, data collection and evaluation, literature search and draft preparation. AHG identified the plant, raised funds, supervised the work and refined the manuscript for publication. NA responsible for critical review, intellectual input in discussion and overall presentation of paper. All authors have read and approved the final manuscript.
Electronic supplementary material
12944_2010_337_MOESM1_ESM.DOC
Additional file 1: Chemical constituents from various parts of Morinda Citrifolia. Following list shows reported chemical constituents isolated from different parts of Morinda citrifolia plant. (DOC 76 KB)
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mandukhail, Su.R., Aziz, N. & Gilani, AH. Studies on antidyslipidemic effects of Morinda citrifolia (Noni) fruit, leaves and root extracts. Lipids Health Dis 9, 88 (2010). https://doi.org/10.1186/1476-511X-9-88
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-511X-9-88