Abstract
Objective
To evaluate the preventive effect of salvianolate (Sal B) on glucose metabolism disorders of dimethylnitrosamine (DMN)-induced cirrhotic rats.
Methods
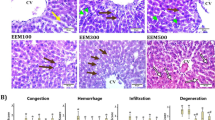
Fifty-five Wistar rats were randomly divided into a control group (n=10) and a cirrhotic group (n=45) according to a random number table. Liver cirrhosis was induced by intraperitoneal administration of DMN. The cirrhotic rats were divided into model, Sal B and metformin groups (n=15), respectively. Rats in the model group were given saline, two treatment groups were given Sal B (50 mg/kg), metformin (150 mg/kg) respectively for 28 consecutive days, while rats in the control group were injected 0.9% saline with same volume of vehicle. Body weight was measured everyday. Insulin sensitivity was determined by euglycemic hyperinsulinemic clamp. Organ index, glucose tolerance test (OGTT), and fasting plasma glucose (FPG), fasting insulin (FINS), hepatic glycogen, hydroxyproline (HYP) and liver function were detected at the end of the treatment. Area under the curve (AUC) for OGTT was calculated. Liver and pancreas histology were determined by histopathological examination with hematoxylin and eosin staining (HE), Sirius Red staining and Masson’s trichrome staining, respectively. Hepatic expression of α-smooth muscle actin (α-SMA) and collagen (Col I) were evaluated by immunohistochemical staining.
Results
Compared with the model group, Sal B significantly increased body and liver weight, liver-body ratio, glucose infusion rate (GIR), FPG, FINS levels and hepatic glycogen at the end of administration (P<0.05 or P<0.01). Meanwhile, Sal B significantly decreased AUC for OGTT, spleen weight, spleen-body ratio, aminotransferase and HYP level (P<0.05 or P<0.01). Sal B was also effective in alleviating necrosis of liver tissue, suppressing fibrosis progression and inhibiting the expression of α-SMA and Col I in liver. Compared with the metformin group, Sal B had advantages in ameliorating FPG, hepatic glycogen, spleen weight, organ index, liver function and cirrhosis (P<0.05). Metformin increased insulin sensitivity more potently than Sal B (P<0.05).
Conclusions
Sal B could improve glucose metabolism in cirrhotic rats by protecting hepatic glycogen reserve, increasing insulin sensitivity, and alleviating pancreatic morphology abnormalities. Sal B was clinically potential in preventing glucose metabolism anomalies accompanied with cirrhosis.
Similar content being viewed by others
References
Nishikawa T, Bellance N, Damm A, Bing H, Zhu Z, Handa K, et al. A switch in the source of ATP production and a loss in capacity to perform glycolysis are hallmarks of hepatocyte failure in advance liver disease. J Hepatol 2014;60:1203–1211.
Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol 2012;27:430–441.
Kwon SY, Kim SS, Kwon OS, Kwon KA, Chung MG, Park DK, et al. Prognostic significance of glycaemic control in patients with HBV and HCV–related cirrhosis and diabetes mellitus. Diabet Med 2005;22:1530–1535.
Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 2007;30:734–743.
Garcia–Compean D, Jaquez–Quintana JO, Lavalle–Gonzalez FJ, Gonzalez–Gonzalez JA, Maldonado–Garza HJ, Villarreal–Perez JZ. Plasma cytokine levels imbalance in cirrhotic patients with impaired glucose tolerance and diabetes mellitus. A prospective study. Ann Hepatol 2014;13:403–410.
Hoehn RS, Singhal A, Wima K, Sutton JM, Paterno F, Steve Woodle E, et al. Effect of pretransplant diabetes on short–term outcomes after liver transplantation: a national cohort study. Liver Int 2015;35:1902–1909.
Hsiang JC, Wong GL, Chan HL, Chan AW, Chim AM, Wong VW. Metabolic syndrome delays HBeAg seroclearance in Chinese patients with hepatitis B. Aliment Pharmacol Ther 2014;40:716–726.
Wlazlo N, van Greevenbroek MM, Curvers J, Schoon EJ, Friederich P, Twisk JW, et al. Diabetes mellitus at the time of diagnosis of cirrhosis is associated with higher incidence of spontaneous bacterial peritonitis, but not with increased mortality. Clin Sci (Lond) 2013;125:341–348.
Garcia–Compean D, Gonzalez–Gonzalez JA, Lavalle–Gonzalez FJ, Gonzalez–Moreno EI, Maldonado–Garza HJ, Villarreal–Perez JZ. The treatment of diabetes mellitus of patients with chronic liver disease. Ann Hepatol 2015;14:780–788.
Liu P, Hu YY, Liu C, Zhu DY, Xue HM, Xu ZQ, et al. Clinical observation of salvianolic acid B in treatment of liver fibrosis in chronic hepatitis B. World J Gastroenterol 2002;8:679–685.
Tao YY, Wang QL, Shen L, Fu WW, Liu CH. Salvianolic acid B inhibits hepatic stellate cell activation through transforming growth factor beta–1 signal transduction pathway in vivo and in vitro. Exp Biol Med (Maywood) 2013;238:1284–1296.
Wang H, Hu YJ, Hong J, Liu P. Effects of salvianolic–acid B on proliferation and TGFβ1 signal transduction of hepatic stellate cells. Chin J Hepatol (Chin) 2002;10:382,394.
Liu P, Liu CH, Wang HN, Hu YY, Liu C. Effect of salvianolic acid B on collagen production and mitogen–activated protein kinase activity in rat hepatic stellate cells. Acta Pharmacol Sin 2002;23:733–738.
Yan X, Zhou T, Tao Y, Wang Q, Liu P, Liu C. Salvianolic acid B attenuates hepatocyte apoptosis by regulating mediators in death receptor and mitochondrial pathways. Exp Biol Med (Maywood) 2010;235:623–632.
Huang M, Wang P, Xu S, Xu W, Xu W, Chu K, et al. Biological activities of salvianolic acid B from Salvia Miltiorrhiza on type 2 diabetes induced by high–fat diet and streptozotocin. Pharm Biol 2015;53:1058–1065.
Ala–Kokko L, Pihlajaniemi T, Myers JC, Kivirikko KI, Savolainen ER. Gene expression of type,and collagens in hepatic fibrosis induced by dimethylnitrosamine in the rat. Biochem J 1987;244:75–79.
Hughey CC, Hittel DS, Johnsen VL. Hyperinsulinemiceuglycemic clamp in the conscious rat. J Vis Exp Jove 2011;48:20.
Salinari S, le Roux CW, Bertuzzi A, Rubino F, Mingrone G. Duodenal–jejunal bypass and jejunectomy improve insulin sensitivity in Goto–Kakizaki diabetic rats without changes in incretins or insulin secretion. Diabetes 2014;63:1069–1078.
Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4–hydroxyproline in biological tissues. Anal Biochem 1981;112:70–75.
Monzillo LU, Hamdy O. Evaluation of insulin sensitivity in clinical practice and in research settings. Nutr Rev 2003;61:397–412.
Tsai MK, Lin YL, Huang YT. Effects of salvianolic acids on oxidative stress and hepatic fibrosis in rats. Toxicol Appl Pharmacol 2010;242:155–164.
Esposito K, Ciotola M, Carleo D, Schisano B, Saccomanno F, Sasso FC, et al. Effect of rosiglitazone on endothelial function and inflammatory markers in patients with the metabolic syndrome. Diabetes Care 2006;29:1071–1076.
Nishikawa H, Osaki Y. Liver cirrhosis: evaluation, nutritional status, and prognosis. Mediators Inflamm 2015;2015:872152.
Winnick JJ, Kraft G, Gregory JM, Edgerton DS, Williams P, Hajizadeh IA, et al. Hepatic glycogen can regulate hypoglycemic counterregulation via a liver–brain axis. J Clin Invest 2016;126:2236–2248.
Senadhi V. Recurrent hypothermia and hypoglycemia as the initial presentation of hepatitis C. Clin Pract 2011;1:e18.
Pfortmueller CA, Wiemann C, Funk GC, Leichtle AB, Fiedler GM, Exadaktylos AK, et al. Hypoglycemia is associated with increased mortality in patients with acute decompensated liver cirrhosis. J Crit Care 2014;29:316 e312–e317.
Aravinthan A, Challis B, Shannon N, Hoare M, Heaney J, Alexander GJ. Selective insulin resistance in hepatocyte senescence. Exp Cell Res 2015;331:38–45.
Chai SY, Pan XY, Song KX, Huang YY, Li F, Cheng XY, et al. Differential patterns of insulin secretion and sensitivity in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease versus patients with type 2 diabetes mellitus alone. Lipids Health Dis 2014;13:7.
Kuroda T, Hirooka M, Koizumi M, Ochi H, Hisano Y, Bando K, et al. Pancreatic congestion in liver cirrhosis correlates with impaired insulin secretion. J Gastroenterol 2015;50:683–693.
Sakata M, Kawahara A, Kawaguchi T, Akiba J, Taira T, Taniguchi E, et al. Decreased expression of insulin and increased expression of pancreatic transcription factor PDX–1 in islets in patients with liver cirrhosis: a comparative investigation using human autopsy specimens. J Gastroenterol 2013;48:277–285.
Lu XL, Dong XY, Fu YB, Cai JT, Du Q, Si JM, Mao JS. Protective effect of salvianolic acid B on chronic pancreatitis induced by trinitrobenzene sulfonic acid solution in rats. Pancreas 2009;38:71–77.
Cheng B, Gong H, Li X, Sun Y, Chen H, Zhang X, et al. Salvianolic acid B inhibits the amyloid formation of human islet amyloid polypeptide and protects pancreatic beta–cells against cytotoxicity. Proteins 2013;81:613–621.
Jeon HK, Kim MY, Baik SK, Park HJ, Choi H, Park SY, et al. Hepatogenous diabetes in cirrhosis is related to portal pressure and variceal hemorrhage. Dig Dis Sci 2013;58:3335–3341.
Ishikawa T, Shiratsuki S, Matsuda T, Iwamoto T, Takami T, Uchida K, et al. Occlusion of portosystemic shunts improves hyperinsulinemia due to insulin resistance in cirrhotic patients with portal hypertension. J Gastroenterol 2014;49:1333–1341.
Zhang X, Harmsen WS, Mettler TA, Kim WR, Roberts RO, Therneau TM, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology 2014;60:2008–2016.
Xin B, Wang XY, Li Y, Qin JH, Ma XJ, Yin JP, et al. Expression and potential role of metastasis–associated protein 1 in the induced carcinogenesis of mouse liver. Chin J Cell Mol Imm (Chin) 2012;28:801–803.
Lees Murdock DJ, Barnett YA, Barnett CR. DNA damage and cytotoxicity in pancreatic beta–cells expressing human CYP2E1. Biochem Pharmacol 2004;68:523–530.
Arai H, Awane N, Mizuno A, Fukaya M, Sakuma M, Harada N, et al. Increasing early insulin secretion compensate adequately for hepatic insulin resistance in CCl4–induced cirrhosis rats. J Med Invest 2010;57:54–61.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Science and Technology Major Project (No. 2014ZX10005001) and the International S&T Cooperation Program of China (No. 2014DFA31440), “Three Year Action Plan” for Development of Traditional Chinese Medicine in Shanghai (No. ZY3CCCX-2-1003), Shanghai Clinical Key Laboratory of Traditional Chinese Medicine (No.14DZ2273200)
Rights and permissions
About this article
Cite this article
Tang, Lr., Tao, Yy., Liu, Ch. et al. Salvianolate Reduces Glucose Metabolism Disorders in Dimethylnitrosamine-Induced Cirrhotic Rats. Chin. J. Integr. Med. 24, 661–669 (2018). https://doi.org/10.1007/s11655-017-2773-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-017-2773-5