Abstract
Objective
To study the effects of allicin on cardiac function and underlying mechanism in rat model of myocardial infarction (MI).
Methods
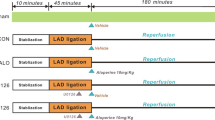
Ninety-four Wistar rats were randomly assigned to 6 groups (n=14–16 per group): sham control group [underwent thoracotomy without left anterior descending (LAD) occlusion and only received an injection of the same amount of citrate buffer], MI control group (subjected to LAD occlusion and only received an injection of same amount of citrate buffer), positive control group (subjected to LAD occlusion and received an injection of diltiazem hydrochloride at the dose of 1.5 mg/kg), and MI + allicin groups (subjected to LAD occlusion and received an injection of allicin at the doses of 1.2, 1.8, and 3.6 mg/kg). All of the drugs were administered intraperitoneally daily for 21 days. The infarct area was measured by myocardial staining. Hematoxylin-eosin staining was used to observe the pathological changes. Cardiac function parameters were assessed by echocardiography. The myocardial apoptotic index was estimated by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining. The expression of Bax and Bcl-2 were detected by quantificational real-time polymerase chain reaction and Western blot.
Results
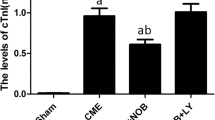
Treatment with allicin could attenuate the myocardial infarct area (P<0.05) and relieve the changes of the myocardium. The left ventricular anterior wall diastolic and systolic thicknesses were increased in the allicin-treated groups (P<0.05), while there was no signifificant difference in the left ventricular posterior wall diastolic and systolic thickness (P>0.05). The left ventricular internal diameter in systole, ejection fraction, fractional shortening, and stroke volume were dramatically elevated in allicin-treated rats (P<0.05). Allicin dose-dependently reduced creatine kinase and lactate dehydrogenase levels (P<0.05). The myocardial apoptotic index was also markedly lowered, and Bax expression was signifificantly decreased, whereas Bcl-2 expression exhibited an opposite trend in allicin-treated rats (P<0.05).
Conclusion
Allicin appears to exert a cardioprotective effect that may be linked to blocking Bcl-2/Bax signaling pathway-denpendent apoptosis, further improving cardiac function.
Similar content being viewed by others
References
Sivaraman V, Yellon DM. Pharmacologic therapy that simulates conditioning for cardiac ischemic/reperfusion injury. J Cardiovasc Pharmacol Ther 2014;19:83–96.
Potenza MA, Nacci C, Gagliardi S, Montaqnani M. Cardiovascular complications in diabetes: lessons from animal models. Curr Med Chem 2011;18:1806–1819.
Fiordaliso F, De Angelis N, Bai A, Cuccovillo I, Salio M, Serra DM, et al. Effect of beta-adrenergic and reninangiotensin system blockade on myocyte apoptosis and oxidative stress in diabetic hypertensive rats. Life Sci 2007;81:951–959.
Dickhout JG, Carlisle RE, Austin RC. Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: endoplasmic reticulum stress as a mediator of pathogenesis. Circ Res 2011;108:629–642.
Mizushige K, Yao L, Noma T, Kiyomoto H, Yu Y, Hosomi N, et al. Alteration in left ventricular diastolic filling and accumulation of myocardial collagen at insulin-resistant prediabetic stage of a type ? diabetic rat model. Circulation 2000;101:899–907.
Wang J, Zhou J, Ding X, Zhu L, Jiang K, Fu M, et al. Qiliqiangxin improves cardiac function and attenuates cardiac remodeling in rats with experimental myocardial infarction. Int J Clin Exp Pathol 2015;8:6596–6606
Ma LN, Li FJ, Chen J, Li YK. Research advances in garlic main active ingredients and their pharmacological effects. Chin Pharm Bull (Chin) 2014;30:760–763.
Hasan N, Yusuf N, Toossi Z, Islam N. Suppression of Mycobacterium tuberculosis induced reactive oxygen species (ROS) and TNF-alpha mRNA expression in human monocytes by allicin. FEBS Lett 2006;580:2517–2522.
Ali M, Thomson M, Afzal M. Garlic and onions: their effect on eicosanoid metabolism and its clinical relevance. Prostaglandins Leukot Essent Fatty Acids 2000;62:55–73.
Sigounas G, Hooker J, Anagnostou A, Steiner M. S-allylmercaptocysteine inhibits cell proliferation and reduces the viability of erythroleukemia, breast, and prostate cancer cell lines. Nutr Cancer 1997;27:186–191.
Hirsch K, Danilenko M, Giat J, Miron T, Rabinkov A, Wilchek M, et al. Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr Cancer 2000;38:245–254.
Xu Y, Yin ZN. Study on antioxidant activity of alliin, alliinase and their mixture in vitro. West Chin J Pharm Sci (Chin) 2011;26:328–330.
Liu C, Cao F, Tang QZ, Yan L, Dong YG, Zhu LH, et al. Allicin protects against cardiac hypertrophy and fibrosis via attenuating reactive oxygen species-dependent signaling pathways. J Nutr Biochem 2010;21:1238–1250.
Chung LY. The antioxidant properties of garlic compounds: allyl cysteine, alliin, allicin, and allyl disulfide. J Med Food 2006;9:205–213.
Ginter E, Simko V. Garlic (Allium sativum L.) and cardiovascular diseases. Bratisl Lek Listy 2010;111:452–456.
Chan JY, Tsui HT, Chang IY, Chan RY, Kwan YW, Chan SW. Allicin protects rat cardiomyoblasts (H9c2 cells) from hydrogenperoxide-induced oxidative injury through inhibiting the generation of intracellular reactive oxygen species. Int J Food Sci Nutr 2014;65:868–873.
Li XH, Li CY, Xiang ZG,Hu JJ, Lu JM, Tian RB, et al. Allicin ameliorates cardiac hypertrophy and fibrosis through enhancing of Nrf2 antioxidant signaling pathways. Cardiovasc Drugs Ther 2012;26:457–465.
Elkayam A, Peleg E, Grossman E, Shabtay Z, Sharabi Y. Effects of allicin on cardiovascular risk factors in spontaneously hypertensive rats. Isr Med Assoc J 2013;15:170–173.
Liu Y, Qi H, Wang Y, Wu M, Cao Y, Huang W, et al. Allicin protects against myocardial apoptosis and fibrosis in streptozotocin-induced diabetic rats. Phytomedicine 2012;19:693–638.
Louis XL, Murphy R, Thandapilly SJ, Yu L, Netticadan T. Garlic extracts prevent oxidative stress, hypertrophy and apoptosis in cardiomyocytes: a role for nitric oxide and hydrogen sulfide. BMC Complement Altern Med 2012;12:140.
Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol 2005;39:813–822.
Penumathsa SV, Thirunavukkarasu M, Koneru S, Juhasz B, Zhan L, Pant R, et al. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J Mol Cell Cardiol 2007;42:508–516.
Kloner RA, Ganote CE, Jennings RB. The "no-reflow" phenomenon after temporary coronary occlusion in the dog. J Clin Invest 1974;54:1496–1508.
Shao L, Wu D, Zhang P, Li W, Wang J, Su G, et al. The significance of micro-thrombosis and fgl2 in no-reflow phenomenon of rats with acute myocardial ischemia/ reperfusion. Clin Appl Thromb Hemost 2013;19:19–28.
Samuel SM, Thirunavukkarasu M, Penumathsa SV, Paul D, Maulik N. Akt/FOXO3a/SIRT1 mediated cardio-protection by n-tyrosol against ischemic stress in rat in vivo model of myocardial infarction: switching gears toward survival and longevity. J Agric Food Chem 2008;56:9692–9698.
Ried K, Frank OR, Stocks NP, Fakler P, Sullivan T. Effect of garlic on blood pressure: a systematic review and metaanalysis. BMC Cardiovasc Disord 2008;8:13.
Arzanlou M, Bohlooli S, Jannati E, Mirzanejad-Asl H. Allicin from garlic neutralizes the hemolytic activity of intra-and extracellular pneumolysin O in vitro. Toxicon 2011;57:540–545.
Foster CR, Daniel LL, Daniels CR, Dalal S, Singh M, Singh K. Deficiency of ataxia telangiectasia mutated kinase modulates cardiac remodeling following myocardial infarction: involvement in fibrosis and apoptosis. PLoS One 2013;8:e83513.
Xia A, Xue Z, Li Y, Wang W, Xia J, Wei T, et al. Cardioprotective effect of betulinic acid on myocardial ischemia reperfusion injury in rats. Evid Based Complement Alternat Med 2014;2014:573745.
Yu W, Shen T, Liu B, Wang S, Li J, Dai D, et al. Cardiac shock wave therapy attenuates H9c2 myoblast apoptosis by activating the AKT signal pathway. Cell Physiol Biochem 2014;33:1293–303.
Jessup M, Brozena S. Heart failure. N Engl J Med 2003;348:2007–2018.
Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, et al. Apoptosis in myocytes in end-stage heart failure. N Engl J Med 1996;335:1182–1189.
Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res 2009;81:457–464.
Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol 1999;277:H1967–H1974.
Del Poeta G, Venditti A, Del Principe MI, Maurillo L, Buccisano F, Tamburini A, et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood 2003;101:2125–2131.
Tatsumi T, Shiraishi J, Keira N, Akashi K, Mano A, Yamanaka S, et al. Intracellular ATP is required for mitochondrial apoptotic pathways in isolated hypoxic rat cardiac myocytes. Cardiovasc Res 2003;59:428–440.
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 1997;275:1132–1136.
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome C from mitochondria blocked. Science 1997;275:1129–1132.
Verma YK, Raghav PK, Raj HG, Tripathi RP, Gangenahalli GU. Enhanced heterodimerization of Bax by Bcl-2 mutants improves irradiated cell survival. Apoptosis 2013;18:212–225.
Xie Z, Koyama T, Suzuki J, Fujii Y, Togashi H, Sawa H, et al. Coronary reperfusion following ischemia: different expression of Bcl-2 and bax proteins, and cardiomyocyte apoptosis. Jap Heart J 2001;42:759–770.
Guttenplan N, Lee C, Frishman WH. Inhibition of myocardial apoptosis as a therapeutic target in cardiovascular disease prevention: focus on caspase inhibition. Heart Dis 2001;3:313–318.
Acknowledgement
We thank Prof. Chen J (Xinjiang Ailexin Parmaceutical Co., Ltd., China), Prof. Chen SK (Xinjiang Ailexin Parmaceutical Co., Ltd., China) and Prof. Li XX (Central Lab, Xinjiang Medical University, China) for providing technical assistance and materials.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Beijing Joint Project Special Funds, and Xinjiang High Technology Research and Development Program (No. 201417111)
Rights and permissions
About this article
Cite this article
Ma, Ln., Li, Ld., Li, Sc. et al. Allicin improves cardiac function by protecting against apoptosis in rat model of myocardial infarction. Chin. J. Integr. Med. 23, 589–597 (2017). https://doi.org/10.1007/s11655-016-2523-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-016-2523-0