Abstract
While it is critical to accurately understand the sources and transformation of sulfate based on time-series analysis, there are limited studies on temporal variation of sulfate in rivers and on rock weathering by sulfuric acids. We conducted a monthly sampling campaign in the Beipan, Nanpan, and Hongshui Rivers over the course of one hydrological year. This study examined seasonal variations in riverine sulfate impacted by the monsoon climate in the upper reaches of the Xijiang River basin. In general, the SO4 2− contents in these rivers dropped from relatively high levels to low values during the high-flow season, in response to increasing discharge. The sulfate was generally enriched in heavy isotopes during the low-flow season compared to the high-flow season. The calculated results indicate that the riverine sulfate was mainly derived from sulfide oxidation, but that evaporite dissolution could be an important source during the low-flow season, based on isotopic evidence. Mine drainage is likely an important source of sulfate to these rivers during the high-flow season due to contributions from fast surface flow, which responds to frequent heavy rain in monsoonal climate regions. A relatively high proportion of HCO3 − was found to be derived from rock weathering by sulfuric acid during the high-flow season when compared to that observed during the low-flow season. The results suggest that approximately one quarter of the HCO3 − in the Hongshui River originated from carbonate weathering by sulfuric acid derived from the oxidation of sulfide. Such information on the specific dual isotopic characteristics of riverine sulfate throughout a hydrological year can provide unique evidence for understanding the temporal variability of sulfate concentrations and weathering processes in rivers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sulfate attracts widespread attention as the dominant form of sulfur in riverine systems. Riverine sulfate generally originates from precipitation and from the weathering of sulfide-bearing minerals and evaporite minerals, as well as from fertilizer and industrial inputs (Yang et al. 1996; Krouse and Mayer 2000; Pawellek et al. 2002; Brenot et al. 2007; Rock and Mayer 2009). There exists a strong linkage between the sulfur cycle and other elemental cycles, such as those of carbon, iron, and nitrogen in the surface environment (Clark and Fritz 1997; Krouse and Mayer 2000; Turchyn and Schrag 2006; Calmels et al. 2007). The temporal and spatial variation of sulfate in river systems can reflect geological characteristics, environmental changes, and anthropogenic inputs (Dogramaci et al. 2001; Jiang et al. 2007). Thus, it is necessary to understand riverine sulfate biogeochemistry in the context of the terrestrial system.
The sulfur and oxygen isotopes of sulfate are powerful tools for identifying the sources and transformation of sulfate in riverine systems (Clark and Fritz 1997). Sulfate derived from the oxidation of reduced sulfur generally inherits the isotopic composition of the reactants during natural processes. The oxygen isotopic composition of sulfate derived from the oxidation of pyrite depends on the ratio of oxygen sources between the water and atmosphere during different oxidation processes that might be controlled by fractionation processes and environmental conditions (Krouse and Mayer 2000; Kohl and Bao 2011). Riverine SO4 2− originating from the dissolution of evaporite minerals is generally enriched in 34S and 18O. Recent studies have shown that the S and O isotopes are useful indicators of the sources and transformations of sulfate in the rivers (Pawellek et al. 2002; Brenot et al. 2007; Calmels et al. 2007; Rock and Mayer 2009). However, there is limited research focusing on the temporal evolution of sulfate and related C–S coupling using the dual isotope approach in large rivers (Brenot et al. 2007; Rock and Mayer 2009; Li et al. 2015).
The Xijiang River is the main tributary of the Pearl River, and has the second largest discharge in China. Previous studies indicate that sulfate in the Xijiang River may derive primarily from oxidation of sulfide minerals in its upper reaches, based on water chemistry and mixing models (Li et al. 2008; Xu and Liu 2010). However, these studies lack isotopic proof to ascertain sulfate sources and implied carbonate weathering. In this study, water chemistry and sulfate isotopes from the upper reaches of the Xijiang River were investigated based on temporally distributed sampling. The main objective was to evaluate the temporal variations of sulfate, as well as the sources of sulfate and its possible implications for the river.
2 Materials and methods
2.1 Sampling site
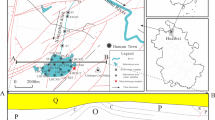
The Xijiang River is located between 21°N and 26°N and 102°E and 115°E. Its drainage basin covers 77.8% of the Pearl River’s entire drainage basin and provides 63.9% of the Pearl’s total discharge. The Xijiang originates in the Maxiong Mountains in Yunnan Province, turns towards the southeast and flows into the South Sea. The length of the Xijiang River is 2075 km. Its drainage basin covers an area of 353,120 km2, and it passes through Guizhou, Guangxi, and Guangdong Provinces. The upper reaches of the Xijiang River include the Nanpan, Hongshui, and Beipan Rivers (Fig. 1). As indicated by Fig. 1, this area is widely covered with Permian and Triassic carbonate rocks (limestones and dolomites). The study area also contains detrital sedimentary rocks (shales, sandstones, and siltstones) and small amounts of magmatic rocks. There exist sparsely distributed coal and metal mines within the investigated area (Li et al. 2008; Xu and Liu 2010). The landscape of the study area is typified by spectacular karst landforms developed in the carbonate bedrock.
A typical monsoonal climate dominates the whole Xijiang River basin in Southwest China. The seasonal variations of air temperature and discharge are in phase; high discharges occur in summer and low discharges occur winter, owing to the Pacific and Indian Ocean monsoons. Winters are cold and dry with little rainfall, while summers are warm and wet and are accompanied by frequent heavy rain. Annual mean temperature ranges from 14 to 22 °C; annual precipitation ranges from 800 to 1200 mm, with 80% occurring during the rainy season, from April to September. Only two small prefecture-level cities are located within the study area. Agriculture is not developed and related land exhibits a scattered distribution (Liu 2007).
2.2 Sampling and analytical techniques
Water samples were collected monthly over a hydrological year from October 2013 to September 2014 from the Beipan, Nanpan, and Hongshui Rivers. Additional water samples were collected from June to July of 2014 in the Nanpan and Beipan Rivers. Meanwhile, rainwater, chemical fertilizer, and groundwater from the gypsum zone were sampled as endmembers. River water samples were filtered through 0.45 µm Millipore nitrocellulose membrane filters. Alkalinity was determined by HCl titration. Major cations (K+, Na+, Ca2+, and Mg2+) were determined using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) with a precision better than 5%, and anions (Cl−, SO4 2−, and NO3 −) were measured by ionic chromatography with a precision of 5%.
Dissolved sulfate was precipitated as BaSO4 for isotopic measurements by adding BaCl2 solution to the samples after acidification using HCl. The precipitate was then filtered, washed, and dried. The values of δ34S-SO4 and δ18O-SO4 were determined using elemental analysis–isotope ratio mass spectrometry (EA-IRMS) and reported using δ notation relative to the Vienna Canyon Diablo Troilite (V-CDT) with a precision better than 0.2‰ and Vienna Standard Mean Ocean Water (V-SMOW) in permil with a precision better than 0.5‰. The measurement of sulfate isotopes was carried out at the Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences. The isotope results are given in δ units, which are defined as:
where R represents the 34S/32S or 18O/16O ratios.
3 Results
3.1 Major ions in the river water
The chemical composition of the water samples from the upper reaches of the Xijiang River was highly variable. The cationic charge (TZ+ = Na+ + K+ + 2Ca2+ + 2 Mg2+) varied from 2245 to 4067 µeq/L with an average value of 3459 µeq/L, which is 1–3 times greater than the average for the large rivers of the world (1125 µeq) (Meybeck 2003). TZ− (HCO3 − + Cl− + 2SO4 2− + NO3 −) varied from 2501 to 4255 µeq/L, with an average of 3589 µeq/L. The normalized inorganic charge balance, NICB = (TZ+ − TZ−)/(TZ+ + TZ−), characterizes the extent of inorganic charge imbalance and generally has values less than ±5%. In the upper reaches of the Xijiang River, the ordering of the cation contents was Ca2+ > Mg2+ > Na+ > K+, and the Ca2+ and Mg2+ contributed, on average, 70% and 22% of TZ+, respectively. The anion contents followed the order HCO3 − > SO4 2− > NO3 − > Cl−, and the HCO3 − and SO4 2− contributed, on average, 70% and 22% of TZ−, respectively.
The concentration of SO4 2− varied from 240 to 752 µmol/L with a mean value of 497 µmol/L for the Beipan River, from 175 to 672 µmol/L with a mean value of 423 µmol/L for the Nanpan River, and from 275 to 679 µmol/L with a mean value of 464 µmol/L for the Hongshui River. For rainwater samples (n = 10) collected from a village in the study area, the concentration of SO4 2− varied from 5 to 27 µmol/L with a mean value of 15 µmol/L. For the Hongshui River, the minimum low-flow value occurred in January (110 m3/s); the maximum high-flow value occurred in September (3769 m3/s) (Fig. 1). River flow can be roughly divided into two regimes for the purposes of this study: low flow (Oct. 2013 through May 2014) and high flow (Jun. 2014 through Sept. 2014).
3.2 Isotopic compositions of sulfate
The isotopic compositions of sulfate were found to range from −4.6‰ to 0.5‰ with a median value of −2.1% for δ34S-SO4 2− in the sampled rivers; for δ18O-SO4 2−, the corresponding range was 3.3‰–12.0‰ and the median value 7.0‰. The mean values of δ34S and δ18O-SO4 2− in the Hongshui River were −1.7‰ and 7.5‰, which were similar to the median values across the study area. The mean values of δ34S and δ18O-SO4 2− in the Beipan River were −2.6‰ and 7.0‰, lower than those observed in the Nanpan and Hongshui Rivers. More depleted sulfate δ18O values were observed in the water samples collected during the wet season (from Jun. to Sept.) compared with those from the dry season (Feb., Apr., and Nov.). Sulfate from rainwater had δ34S-SO4 2− values of 1.4‰ ± 0.7‰ and δ18O-SO4 2− values of 8.9‰ ± 2.2‰. Chemical fertilizer returned δ34S-SO4 2− values of 14.6‰ ± 2.1‰ and δ18O-SO4 2− values of 32.9‰ ± 4.3‰. The δ34S- and δ18O-SO4 2− of groundwater from the gypsum zone were 25.7‰ ± 1.5‰ and 11.2‰ ± 1.2‰, respectively.
4 Discussion
4.1 Seasonal variations in sulfate and isotopic compositions
In general, there were relatively low contents of the ions Ca2+, HCO3 −, SO4 2−, Mg2+, and K+ during the high-flow season compared with the low-flow season in sampled rivers, indicating a dilution effect. There was a clear inverse relationship between discharge and conductivity during the hydrological year in the Hongshui River (Fig. 2a), suggesting that the dissolved load is distinctly impacted by dilution processes. However, NO3 − and Cl− did not show negative relationships with discharge in the Hongshui River, indicating complex mixing from various anthropogenic inputs, such as chemical fertilizer, domestic waste, and industrial effluent. Meanwhile, relatively high contents of sulfate were found in some water samples collected from the Beipan River during the high-flow season, which might reflect drought-condition reservoir evaporation and substantial inputs from mine drainage and industrial activities, given the coal and metal mines in the study area (Hong et al. 1993; Liu 2007; Li et al. 2008; Yuan 2014). In general, a distinct dilution of sulfate during the high-flow season was evidenced by the inverse relationship between discharge and sulfate, such as that seen in the Hongshui River (Fig. 2b).
In the upper reaches of the Xijiang River, there was a non-significant inverse relationship between the reciprocal of SO4 2− concentration and the values of δ34S-SO4 2− and δ18O-SO4 2− (Fig. 3). A wide range of river water 1/SO4 2− values were measured during the high-flow season, varying from 1.4 to 4.2, and the sulfate was enriched in light sulfur and heavy oxygen isotopes. The 1/SO4 2− values fell within a narrower range, 1.6 to 2.3, during the low-flow season, when the sulfate was enriched in heavy sulfur and oxygen isotopes. However, more negative δ34S and δ18O values of sulfate were observed in the water samples during the high-flow season (from Jun. to Sept.) compared with the low-flow season (Fig. 3). In general, the seasonal variations of isotopic compositions reflected different hydrological conditions. Sulfate originating from mine activities and industrial effluents can be transported through surface flow during the high-flow season. Similar observations have been reported in the Jialing River basin (Li et al. 2011a, b).
4.2 Sulfate sources constrained by isotopes
The dual isotope approach (δ34S-SO4 2− and δ18O-SO4 2−) presents an opportunity to elucidate the sources and fate of sulfate in various rivers (Calmels et al. 2007; Rock and Mayer 2009; Turchyn et al. 2013; Li et al. 2014). Distinct isotopic “fingerprint” signatures can be used to characterize different sulfate sources (Krouse and Grinenko 1991), including atmospheric deposition; evaporite dissolution (largely gypsum, CaSO4·2H2O, or anhydrite, CaSO4); sulfide oxidation (largely pyrite, FeS2); and anthropogenic inputs, such as chemical fertilizer, domestic waste, industrial effluent, and acid mine drainage (Krouse and Mayer 2000; Brenot et al. 2007; Rock and Mayer 2009). The specific dual isotopic characteristics of riverine sulfate are of unique value in understanding the hydrological year variability of sources in the studied watershed, especially in light of its monsoon climate.
Low pH values and high sulfate contents in rainwater have been found in the industrialized areas of China as a result of anthropogenic activity (Larssen et al. 1999). Southwest China is one of the areas that is most affected by acid rain. The δ34S-SO4 2− values measured in this study from precipitation are characteristic of anthropogenic emissions, especially in industrialized areas. Rainwater samples were collected in situ during the sampling period and had a median value of 30 μmol/L SO4 2−, lower than that observed in Guiyang rainwater (80 μmol/L SO4 2−; Han and Liu 2006) or in the studied river water. The isotopic values of sulfate from rain in this study are similar to those from the Sichuan basin (Li et al. 2006), where reported δ34S-SO4 2− values of rainwater ranged from 1.7‰ to 5.6‰ (averaging 3.9‰). It has been reported that the δ34S-SO4 2− values of rainwater ranged from −8.1‰ to −5‰ in Guiyang (Jiang et al. 2007; Liu 2007). The values of δ18O-SO4 2− in atmospheric sulfate generally reflect enrichment in heavy 18O, ranging from 7‰ to 17.2‰ in previous studies (Krouse and Mayer 2000; Jenkins and Bao 2006; Brenot et al. 2007), which have also shown that the range of δ18O-SO4 2− values was between 10‰ and 20‰ for atmospheric sulfate. Atmospheric deposition is likely one of the important sources of sulfate in the upper reaches of the Xijiang River based on the frequent acid deposition conditions in the study area (Jiang et al. 2007; Liu 2007) and the δ34S-SO4 2− versus δ18O-SO4 2− diagram (Fig. 4).
The sulfate derived from evaporite dissolution is typically enriched in heavy sulfur and oxygen isotopes (34S and 18O) and thus plots within the upper right-hand quadrant. It exhibits \(\updelta^{34} {\text{S}}_{{{\text{SO}}_{4} }}\) values ranging from 8‰ to 35‰ and \(\updelta^{18} {\text{O}}_{{{\text{SO}}_{4} }}\) values ranging from 6‰ to 20‰ (Claypool et al. 1980; Turchyn and Schrag 2006), which typically vary with geological age. The \(\updelta^{34} {\text{S}}_{{{\text{SO}}_{4} }}\) values of gypsum deposited during the Late Proterozoic through the Early Cambrian range from 21‰ to 32‰ (Strauss 1997), while \(\updelta^{18} {\text{O}}_{{{\text{SO}}_{4} }}\) values range from 10‰ to 20‰ (Turchyn et al. 2013). Sulfate derived mainly from Triassic evaporites yields values of δ34S and δ18O ranging from 14.5‰ to 32.5‰ and from 13‰ to 15‰, respectively (Strauss 1997; Goldberg et al. 2005). Within the study area, we collected water samples that inherited evaporite isotopic characteristics. The isotopic values of sulfate were higher than those from the river waters; in particular, the δ34S-SO4 2− values were above +20‰.
Shale and sulfide minerals are widely distributed in the investigated catchment; thus, sulfide oxidation could be an important source of sulfate contributions to the Xijiang River. In a previous study, δ34S values mainly ranged from −27‰ to 15‰ for sulfide minerals situated in southwest Guizhou Province (Yuan 2014). In general, sulfate derived from oxidation of sulfide in Guizhou Province has δ34S values ranging from 5‰ to 10‰ (Liu 2007). Coal has δ34S values that mainly range from −7‰ to 5‰ in Guizhou Province (Hong et al. 1993). Coal mines are widely distributed in the Beipan and Nanpan River basins. Mine drainage in Guizhou Province, has δ34S values close to −13‰ (Jiang et al. 2007). In general, δ34S-SO4 2− generally inherits the isotopic composition of the reduced sulfur (Krouse and Mayer 2000; Liu 2007), while the ratio of oxygen between atmosphere and water in sulfate during pyrite oxidation might depend on the various oxidation pathways and environmental conditions (Kohl and Bao 2011). In general, two main sulfide oxidation processes are present under natural conditions (Taylor et al. 1984; van Everdingen and Krouse 1985; van Stempvoort and Krouse 1994). The first major reaction of pyrite oxidation is as follows:
where 7/8 of the oxygen in the sulfate is contributed by molecular atmospheric oxygen, while the remaining 1/8 oxygen comes from water. The resulting sulfate is enriched in the heavy sulfur isotope (34S) and the light oxygen isotope (16O). The second reaction is the oxidation of pyrite by ferric iron:
where the oxygen in the sulfate is derived exclusively from water. Compared to Reaction 2, ferric iron can rapidly oxidize pyrite under anaerobic and abiotic conditions via Reaction 3, respectively. Either Reactions 2 or 3 may dominate, which demonstrates the complexity of oxygen sources in riverine sulfate. In addition to the two primary oxygen sources, the δ18O value of sulfate is influenced by isotopic fractionation effects. According to the second equation (van Everdingen and Krouse 1985; van Stempvoort and Krouse 1994), we could calculate the δ18O of dissolved sulfate using the fraction of O2 versus H2O and their respective enrichment factors. The large contrast in the oxygen isotopic composition between the atmosphere and meteoric water can potentially allow identification of pyrite oxidation pathways. The isotopic compositions of atmospheric oxygen and meteoric water are approximately 23.5‰ and <0‰, respectively (Kroopnick and Craig 1972). The enrichment factors of \(\upvarepsilon^{18} {\text{O}}_{{{\text{SO}}_{4} {-} {\text{H}}_{2} {\text{O}}}}\) and \(\upvarepsilon^{18} {\text{O}}_{{{\text{SO}}_{4} {-} {\text{O}}_{2} }}\) can be assigned values of 4.1‰ and −11.4‰ (Taylor et al. 1984; van Everdingen and Krouse 1985; van Stempvoort and Krouse 1994). Within the study area, the δ18O-H2O in river waters ranges approximately from −10‰ to −8‰ (Li et al. 2015); therefore, the δ18O-SO4 2− values would be −5‰ to 9‰. Of course, there are the above-mentioned uncertainties of oxygen isotopic composition during sulfide oxidation. In general, sulfate derived from the oxidation of coal is enriched in the heavy isotopes of oxygen, due to oxidation by oxygen. However, sulfate derived from oxidation of sulfide minerals by iron is enriched in the light oxygen isotope. Meanwhile, sulfate derived from the dissolution of gypsum generally has δ18O-SO4 2− values above +10‰ (Turchyn and Schrag 2006), suggesting that oxidation of reduced sulfur compounds is a major source of riverine sulfate in the present study. Jiang et al. (2007) and Li et al. (2011a, b) described similar observations in the Wujiang and Jialingjiang Rivers, respectively, and proved that most riverine sulfate derived from sulfide oxidation.
The values of δ34S and δ18O for sulfate of anthropogenic origin range from 0‰ to 10‰ and from 5‰ to 20‰, respectively (Longinelli and Cortecci 1970; Krouse and Grinenko 1991; Jådrysek 2000). The δ34S values of domestic detergents widely used in homes in the Sichuan Basin range from 15.2‰ to 17.2‰ (Li et al. 2006), while in Spain these values range from 8.5‰ to 13.6‰ (Vitòria et al. 2004). The δ34S and δ18O values of fertilizers widely used in Spain range from −6.5‰ to +21.4‰ and from 7.7‰ to +16.5‰, respectively (Vitòria et al. 2004). Chemical fertilizers have relatively high δ34S-SO4 2− and δ18O-SO4 2− values, which are far above those observed in river waters. Thus, the contribution of chemical fertilizers appears to be of minor importance for riverine sulfate.
The sulfur and oxygen isotopic compositions of sulfate in the upper reaches of the Xijiang River suggest at least four endmembers—atmospheric deposition, oxidation of reduced sulfur, evaporite dissolution, and anthropogenic inputs (Fig. 4). As indicated by Fig. 4, oxidation of reduced sulfur should be a major source of sulfate for these rivers. The riverine SO4 2− was characterized by relatively high values of δ34S-SO4 2− and δ18O-SO4 2− during the low-flow season, indicating that more sulfate originated from evaporite dissolution at that time than during the high-flow season. Sulfate was characterized by relatively low δ34S-SO4 2− and δ18O-SO4 2− values during the high-flow season, suggesting greater contributions from sulfide oxidation and atmospheric deposition. More sulfate derived from the oxidation of sulfide from mines and soil is transported via surface flow into rivers during the high-flow season, which might account for shifts in isotopic values and seasonal variability of sulfate in the rivers. A similar pattern—lower δ34S-SO4 2− values and higher δ18O-SO4 2− values in the rainy season—was reported in the Yangtze River (Li et al. 2011b).
4.3 Calculated contributions from different sulfate sources
The various riverine sulfate sources can be quantified based on the above discussion of potential sources of sulfate to the upper reaches of the Xijiang River. Potential contributions from chemical fertilizers were ignored in this study, due to the large differences in isotopic compositions between fertilizer and the sulfate in the river. Additionally, sulfate is not a major component of most chemical fertilizers. The chemical characteristics of the river water samples of the Hongshui River were used in our calculations. We assigned δ34S-SO4 2− and δ18O-SO4 2− values of 1.4‰ and 8.9‰ for rainwater, −13.0‰ and 9.0‰ for the oxidation of coal, 8.0‰ and −2.0‰ for the oxidation of sulfide, and 25.7‰ and 11.2‰ for evaporite dissolution, respectively. The rainwater from villages in the study area contained small amounts of sulfate, with a median value of 30 μmol/L. This concentration is lower than the 90 μmol/L observed in rainwater of urban areas in southwestern China (Jiang et al. 2007). Here, we assigned the atmospheric source of sulfate a concentration of 60 μmol/L after consideration of the evaporation effect and of the limited rainwater samples collected in the studied area. Thus, the contribution of sulfate to the river from rainwater ranged from 8% to 34% in these rivers. The contributions from different endmembers were calculated as follows (Li et al. 2014):
where (a + b) is the proportion of the total sulfate derived from oxidation of sulfide with different isotopic characteristics. δ34S*river and δ18O*river represent isotopic values of sulfate after correction for rain input. Water flux in the high-flow and low-flow seasons make up 80% and 20% of the total flux, respectively, in the upper reaches of the Xijiang River (the mean annual runoff is 687 × 108 m3; Pearl River Water Resources Commission of the Ministry of Water Resources, http://www.pearlwater.gov.cn/). The calculations show that in the Hongshui River—in the upper reaches of the Xijiang River—sulfide oxidation contributed 65% of the sulfate in the low-flow season and 80% in the high-flow season; rainwater contributed 12%–15% of the sulfate to the Hongshui River. On the other hand, evaporite dissolution became increasingly significant during the low-flow season. The calculated data are consistent with the above discussion of changes in the various sources of riverine sulfate between the two seasons in the upper reaches of the Xijiang River. Of course, the calculated results might over- or underestimate the contribution from oxidation of sulfide and gypsum since we ignored anthropogenic sources in the calculations, due to their assumed relatively minor contribution. Ultimately, the calculated contribution of sulfate from the oxidation of sulfide in the Hongshui River is in agreement with previous studies in Spence and Telmer (2005); Calmels et al. (2007) and Li et al. (2011a, b, 2014). These studies suggest that high proportions of sulfate are derived from the oxidation of sulfide in various riverine systems.
4.4 Implications for the carbonate weathering budget
Previous studies have suggested that sulfuric acid is an important weathering agent for rock weathering in various basins, due to the wide distribution of sulfide minerals over the Earth’s surface (Spence and Telmer 2005; Calmels et al. 2007; Li et al. 2008, 2011a, b, 2014). Oxidation of sulfide minerals produces acid, and the acid reacts with rock, especially carbonates. Hong et al. (1993) showed that large amounts of sulfides are present in the coal strata interbedded with carbonates in South China. In such a system, reactions between sulfuric acid and carbonate rock should occur widely. In this study, approximately 77% of the sulfate was derived from the oxidation of sulfide, which suggests that a large amount of acid is produced in the upper reaches of the Xijiang River. However, the acid would be neutralized by carbonate rock and produce alkalinity for rivers. In this study, carbonate weathering accounted for approximately 23% of HCO3 − during the low-flow season and 26% of HCO3 − during the high-flow season, with negligible silicate weathering by sulfuric acid. These proportions are higher than those in the Yalong River (Li et al. 2014) and lower than those in the Beipan River (Li et al. 2008). The calculated proportion has some uncertainties due to dissimilarities among the assigned endmembers, uncertainties in the anthropogenic input, the neglect of biological processes, and errors introduced through multiple computations. Nonetheless, the large amount of HCO3 − produced by the reaction of carbonate rock and sulfuric acid should be considered carefully in the context of regional and global carbon cycling.
5 Conclusions
In this study, sources and seasonal variations of sulfate were identified using stable isotopic analysis of samples collected monthly in the Beipan, Nanpan, and Hongshui Rivers over the course of a hydrological year. We examined the effects of seasonal variations (impacted by the monsoon climate) on the sulfate sources of the upper reaches of the Xijiang River. The distinct seasonal variations in sulfate in the rivers showed lower SO4 2− contents during the high-flow season, suggesting a dilution effect. In general, the sulfate was generally enriched in light isotopes during the high-flow season compared to the low-flow season, which might reflect anthropogenic inputs, such as mine drainage that follows fast surface flow in response to the frequent heavy rains in the study area. The calculated results indicate that the riverine sulfate was mainly derived from sulfide oxidation, whereas evaporation dissolution could have been important during the low-flow season, based on the isotopic evidence. The results further suggest that a large amount of sulfate in the Hongshui River was derived from sulfide oxidation, including 65% in the low-flow season and 80% in the high-flow season. Approximately 23% of the HCO3 − in the low-flow season and 26% of the HCO3 − in the high-flow season originated from carbonate weathering by sulfuric acid. This suggests that carbonate weathering by sulfuric acid should be incorporated into the carbon budget of this riverine system. In sum, information on the dual isotopic characteristics of riverine sulfate can be uniquely helpful in understanding seasonal variability in sulfate sources and weathering processes in these rivers.
References
Brenot A, Carignan J, France-Lanord C, Benoit M (2007) Geological and land use control on δ34S and δ18O of river dissolved sulfate: the Moselle river basin, France. Chem Geol 244(s1–2):25–41
Calmels D, Gaillardet J, Brenot A, France-lanord C (2007) Sustained sulfide oxidation by physical erosion processes in the Mackenzie River Basin: climatic perspectives. Geology 35(11):1003–1006
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York, p 328
Claypool GE, Holser WT, Kaplan IR, Sakai H, Zak I (1980) The age curves for sulfur and oxygen isotopes in marine sulfate and their mutual interoretation. Chem Geol 28:199–260
Dogramaci SS, Herczeg AL, Schiff SL, Bone Y (2001) Controls on δ34S and δ18O of dissolved sulfate in aquifers of the Murray Basin, Australia and their use as indicators of flow processes. Appl Geochem 16:475–488
Goldberg T, Poulton SW, Strauss H (2005) Sulphur and oxygen isotope signatures of late Neoproterozoic to early Cambrian sulphate, Yangtze Platform, China: diagenetic constraints and seawater evolution. Precambrain Res 137(3):223–241
Han GL, Liu CQ (2006) Strontium isotope and major ion chemistry of the rainwaters from Guiyang, Guizhou Province, China. Sci Total Environ 364(1–3):165–174
Hong YT, Zhang HB, Zhu X (1993) Sulfur isotopic characteristics of coal in China and sulfur isotopic fractionation during coal-burning process. Acta Geochim 12(1):51–59
Jådrysek MO (2000) Oxygen and sulphur isotope dynamics in the SO4 2− of an urban precipitation. Water Air Soil Pollut 117(1):15–25
Jenkins KA, Bao H (2006) Multiple oxygen and sulfur isotope compositions of atmospheric sulfate in Baton Rouge, LA, USA. Atmos Environ 40(24):4528–4537
Jiang YK, Liu CQ, Tao FX (2007) Sulfur isotope composition characters of Wujiang river water in Guizhou province. Adv Water Sci 18(4):558–565 (in Chinese)
Kohl I, Bao H (2011) Triple-oxygen-isotope determination of molecular oxygen incorporation in sulface produced during abiotic pyrite oxidation (pH = 2–11). Geochim Cosmochim Acta 75(7):1785–1798
Kroopnick P, Craig H (1972) Atmospheric oxygen: isotopic composition and solubility fractionation. Science 175(175):54–55
Krouse HR, Grinenko VA (1991) Stable isotopic: natural and anthropogenic sulfur in the environment. SCOPE, 43, vol 440. Wiley, Chichester, pp 177–265
Krouse HR, Mayer B (2000) Sulphur and oxygen isotopes in sulfate. In: Cook PG, Herczeg AL (eds) Environmental tracers in subsurface hydrology. Kluwer, Boston, pp 195–231
Larssen T, Seip HM, Semb A, Mulder J, Muniz IP, Vogt RD, Lydersen E, Angell V, Tang D, Eilertsen O (1999) Acid deposition and its effects in China: an overview. Environ Sci Policy 2(1):9–24
Li XD, Masuda H, Kusakabe M, Yanagisawa F, Zeng HA (2006) Degradation of groundwater quality due to anthropogenic sulfur and nitrogen contamination in the Sichuan Basin, China. Geochem J 40(4):309–332
Li SL, Calmels D, Han GL, Gaillardet J, Liu CQ (2008) Sulfuric acid as an agent of carbonate weathering constrained by δ13CDIC: examples from southwest China. Earth Planet Sci Lett 270(3):189–199
Li SL, Liu CQ, Patra SJ, Wang FS, Wang BL, Yue FJ (2011a) Using a dual isotopic approach to trace sources and mixing of sulfate in Changjiang Estuary, China. Appl Geochem 26(3):S210–S213
Li XD, Liu CQ, Liu XL, Bao LR (2011b) Identification of dissolved sulfate sources and the role sulfuric acid in carbonate weathering using dual-isotopic data from the Jialing River, Southwest China. J Asian Earth Sci 42(3):370–380
Li SL, Chetelat B, Yue FJ, Zhao ZQ, Liu C-Q (2014) Chemical weathering processes in the Yalong River draining the eastern Tibetan Plateau, China. J Asian Earth Sci 88:74–84
Li X, Gan Y, Zhou A, Liu Y (2015) Relationship between water discharge and sulfate sources of the Yangtze River inferred from seasonal variations of sulfur and oxygen isotopic compositions. J Geochem Explor 153:30–39
Liu CQ (2007) Biogeochemical processes and cycling of nutrients in the earth’s surface: chemical erosion and nutrient cycling in karstic catchments, Southwest China. Science Press, Beijing, p 608 (in Chinese)
Longinelli A, Cortecci G (1970) Isotopic abundance of oxygen and sulfur in sulfate ions from river water. Earth Planet Sci Lett 7(4):376–380
Meybeck M (2003) Global occurrence of major elements in rivers. In: Drever JI (ed) Treatise on geochemistry, surface and ground water, weathering, and soils. Elsevier, pp 207–223
Pawellek F, Frauenstein F, Veizer J (2002) Hydrochemistry and isotope geochemistry of the upper Danube River. Geochim Cosmochim Acta 66(21):3839–3853
Rock L, Mayer B (2009) Identifying the influence of geology, land use, anthropogenic activities on riverine sulfate on a watershed scale by combining hydrometric, chemical and isotopic approaches. Chem Geol 262(3–4):121–130
Spence J, Telmer K (2005) The role of sulfur in chemical weathering and atmospheric CO2 fluxes: evidence from major ions, δ13CDIC, and δ34SSO4 in rivers of the Canadian Cordillera. Geochim Cosmochem Acta 69(23):5441–5458
Strauss H (1997) The isotopic composition of sedimentary sulfur through time. Palaeogeogr Palaeoclimatol Palaeoacol 132(1):97–118
Taylor BE, Wheeler MC, Nordstrom DK (1984) Stable isotope geochemistry of acid mine drainage: experimental oxidation of pyrite. Geochim Cosmochim Acta 48(12):2669–2678
Turchyn AV, Schrag DP (2006) Cenozoic evolution of the sulfur cycle: insight from oxygen isotopes in marine sulfate. Earth Planet Sci Lett 241(3–4):763–779
Turchyn AV, Tipper ET, Galy A, Lo J-K, Bickle MJ (2013) Isotope evidence for secondary sulfide precipitation along the Marsyandi River, Nepal, Himalayas. Earth Planet Sci Lett 374(4):36–46
van Everdingen RO, Krouse HR (1985) Isotope composition of sulfates generated by bacterial and abiological oxidation. Nature 315:395–396
van Stempvoort DR, Krouse HR (1994) Controls of δ18O in sulfate: review of experimental date and application to specific environments. In: Alpers CA, Blowes DW (eds) Environmental geochemistry of sulfide oxidation. American chemical society symposium series, vol 550. ACS Publications, Washington DC, pp 446–480
Vitòria L, Otero N, Soler A, Canals A (2004) Fertilizer characterization: isotopic data (N, S, O, C and Sr). Environ Sci Technol 38(12):3254–3262
Xu ZF, Liu C-Q (2010) Water geochemistry of the Xijiang basin rivers, South China: chemical weathering and CO2 consumption. Appl Geochem 25(10):1603–1614
Yang C, Telmer K, Veizer J (1996) Chemical dynamics of the “St. Lawrence” riverine system: δDH2O, δ18OH2O, δ13CDIC, δ34Ssulfate, and dissolved 87Sr/86Sr. Geochim Cosmochim Acta 60(5):851–866
Yuan L (2014) Geological genesis of the high sulfur coal from southwestern Guizhou: analysis of pyrite and sulfur isotope. Mater Degree thesis. China University of Geosciences
Acknowledgements
This work was financially supported by the Ministry of Science and Technology of China through Grant Nos. 2016YFA0601000 and 2013CB956700 and National Natural Science Foundation of China (Grant Nos. 41422303, 41130536 and 41625006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, J., Li, S., Zhong, J. et al. Sulfate sources constrained by sulfur and oxygen isotopic compositions in the upper reaches of the Xijiang River, China. Acta Geochim 36, 611–618 (2017). https://doi.org/10.1007/s11631-017-0175-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-017-0175-1