Abstract
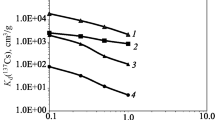
This study examines the use of intact samples as an alternative to powder in conventional batch sorption studies to determine the distribution coefficient (K d). Stable cesium (133Cs) and strontium (87Sr) were used under specified geochemical conditions to compare the K d values of powder and block pumice tuff samples. The aim of the study was to infer any K d difference under laboratory and field conditions. K d values for block samples were found to be less than one order of magnitude lower than powder materials for both Cs and Sr on fresh tuff, and more than one order of magnitude lower in oxidized tuff. Destruction of micropores in oxidized tuff was estimated to be mainly responsible for reducing K d values in oxidized tuff. However, approximately one order of magnitude difference in K d values indicates that homogenously prepared intact samples can be used for sorption coefficient measurement at closer to in situ conditions. Pore size distribution analysis using mercury intrusion porosimetry revealed that lower K d values on block samples result from lower surface area available as sorption sites due to inaccessible closed pores in the intact solid.





Similar content being viewed by others
References
Allen HE, Chen YT, Li Y, Huang CP, Sanders PF (1995) Soil partition coefficients for Cd by column desorption and comparison to batch adsorption measurements. Environ Sci Technol 29:1887–1891. doi:10.1021/es00008a004
Chang TW, Wang MK (2002) Assessment of sorbent/water ratio effect on adsorption using dimensional analysis and batch experiments. Chemosphere 48:419–426. doi:10.1016/S0045-6535(02)00053-X
Du YJ, Hayashi S (2006) Experimental study of factors controlling sorption of heavy metals on Ariake clay and implication for practice. Mar Georesour Geotech 24:103–118. doi:10.1080/10641190600704475
Esa P (2014) Sorption of cesium on intact rocks, University of Helsinki, Department of Chemistry, Laboratory of Radiochemistry, 2013–2014 Working report, 1–25
Hu Q, Mao X (2012) Application of laser ablation-inductively coupled plasma-mass spectrometry to studies of chemical diffusion, sorption, and transport in natural rock. Geochem J 46:459–475. doi:10.2343/geochemj.2.0225
Igarashi T, Mahara Y, Ashikawa N, Okamura M (1998) Evaluation of radioactive strontium distribution coefficient by analyzing background stable strontium. J Nucl Sci Tech 35:190–197. doi:10.1080/18811248.1998.9733844
Iida Y, Tanaka T, Yamaguchi T, Nakayama S (2001) The solubility of metallic selenium under anoxic conditions. MRS Proc. 663:1143–1149. doi:10.1557/PROC-663-1143
JAEA (2014) Japan atomic energy agency sorption database (JAEA-SDB). http://www.jaea.go.jp/04/tisou/english/database/database.html
Kobayashi T, Sasaki T, Takagi I, Moriyama H (2009) Zirconium solubility in ternary aqueous system of Zr(IV)-OH-carboxylates. J Nucl Sci Technol 46:142–148. doi:10.1080/18811248.2007.9711515
Lee CP, Wu MC, Tsai TL, Wei HJ, Men LC, Lin TY (2012) Comparative study on retardation behavior of Cs in crushed and intact rocks: two potential repository host rocks in the Taiwan area. J Radioanal Nucl Chem 293:579–586. doi:10.1007/s10967-012-1684-3
Li MH, Wang TH, Teng SP (2009) Experimental and numerical investigations of effect of column length on retardation factor determination: a case study of cesium transport in crushed granite. J Hazard Mater 162:530–535. doi:10.1016/j.jhazmat.2008.05.076
Limousin G, Gaudet JP, Charlet L, Szenknect S, Barthes V, Krimissa M (2007) Sorption isotherms: a review on physical bases, modeling and measurement. Appl Geochem 22:249–275. doi:10.1016/j.apgeochem.2006.09.010
Maclntyre WG, Stauffer TB, Antworth CP (1991) A comparison of sorption coefficients determined by batch, column, and box methods on a low organic carbon aquifer material. Ground Water 29:908–913. doi:10.1111/j.1745-6584.1991.tb00578.x
OECD (2001) Using Thermodynamic sorption models for guiding radioelement distribution coefficient (Kd) investigations: A status report, Part-Iⅈ Nuclear Energy Agency, Organization for Economic Co-operation and Development. http://www.oecd-ilibrary.org/nuclear-energy/
Ohnuki T, Yoshida T, Ozaki T, Naofumi K, Sakamoto F, Nankawa T, Suzuki Y, Francis AJ (2009) Modeling of the interaction of Pu(VI) with the mixture of microorganism and clay. J Nucl Sci Technol 46:55–59. doi:10.1021/es061207g
Oyama T, Inohara Y, Nagaoka T (2007) Development of the investigation and evaluation method for geochemical condition of underground- In situ redox conditions and its prediction at the Rokkasho site. Civil Engineering Research Laboratory Report No. N07001 (in Japanese with English abstract)
Qin H, Yokoyama Y, Fan Q, Iwatani H, Tanaka K, Sakaguchi A, Kanai Y, Zhu J, Onda Y, Takahashi Y (2012) Investigation of cesium adsorption on soil and sediment samples from Fukushima Prefecture by sequential extraction and EXAFS technique. Geochem J 46:297–302. doi:10.2343/geochemj.2.0214
Rajib M, Sasaki T, Kobayashi T, Miyauchi Y, Takagi I, Moriyama H (2011) Analysis of sorption behavior of cesium and iodide ions on pumice tuff. J Nucl Sci Technol 48:950–957. doi:10.1080/18811248.2011.9711781
Rajib M, Oguchi CT, Sasaki T, Kobayashi T (2015) Strontium dissolution effect on the adsorption experiment with rhyolitic pumice tuff. Geochem J 49:539–548. doi:10.2343/geochemj.2.0383
Rajib M, Kobayashi T, Oguchi CT, Sasaki T (2016) Oxidation of solid phase and ionic strength effect to the cesium adsorption on pumice tuff. J Geosci Environ Prot 4:64–73. doi:10.4236/gep.2016.42008
Rogers PSZ, Meijer A (1993) Dependence of radionuclide sorption on sample grinding, surface area, and water composition, LA-UR-93-270. Proc Int High Lev Radioact Waste Manag Conf 2:1509–1516
Rylea JF, Serne RJ and Rai D (1980) Methods for determining radionuclide retardation factors: Status report. US Department of Energy, PNL-3349, UC-70
Sasaki T (2005) The site investigation at the next Rokkasho disposal facility- Disposal at around 50–100 m depth, Proceedings of GLOBAL 2005, Tsukuba, Oct 9–13, p. 069
Sasaki T, Terakado Y, Kobayashi T, Takagi I, Moriyama H (2007) Analysis of sorption behavior of cesium ion on mineral components of granite. J Nucl Sci Technol 44:641–648. doi:10.1080/18811248.2007.9711852
Wang TH, Li MH, Teng SP (2009) Bridging the gap between batch and column experiments: A case study of Cs adsorption on granite. J Hazard Mater 161:409–415. doi:10.1016/j.jhazmat.2008.03.112
Widerstand H, Byegård J, Selnert E, Skålberg M, Höglund S, Gustafsson E (2010) Long Term Sorption Diffusion Experiment (LTDE-SD) supporting laboratory program—Sorption diffusion experiments and rock material characterization, Swedish Nuclear Fuel and Waste Management Co. ISSN 1402–3091, SKB R-10-66
Wise WR (1993) Effects of laboratory-scale variability upon batch and column determinations of nonlinearly sorptive behavior in porous media. Water Resour Res 29:1944–7973. doi:10.1029/93WR00967
Wu MC, Lee CP, Tsai SC, Liu CW, Pan CH, Tsai TL, Wei HJ, Men LC (2015) Study on sorption and diffusion of Sr in crushed and intact basalt and granite investigated in through-diffusion experiments. J Radioanal Nucl Chem 304:435–441. doi:10.1007/s10967-014-3889-0
Xia X, Iijima K, Kamei G, Shibata M (2006) Comparative study of cesium sorption on crushed and intact sedimentary rock. Radiochim Acta 94:683–687. doi:10.1524/ract.2006.94.9.683
Xiao C, Zhang A (2016) Synthesis and characterization of a cesium-selective macroporous silica-based supramolecular recognition material with high stability. J Radioanal Nucl Chem 307:713–723. doi:10.1007/s10967-015-4171-9
Xiao C, Zhang A, Chai Z (2014) Synthesis and characterization of a novel organic–inorganic hybrid supramolecular recognition material and its selective adsorption for cesium. J Radioanal Nucl Chem 299:699–708. doi:10.1007/s10967-013-2798-y
Acknowledgements
Authors are thankful to Japan Nuclear Fuel Limited (JNFL) for providing samples. They are also grateful to Dr. Takayuki Sasaki and Dr. Taishi Kobayashi from Department of Nuclear Engineering, Kyoto University for solution analysis using ICP-MS, as well as for their critical comments and suggestions. Efforts of anonymous reviewers to upgrade the manuscript to a publishable one are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajib, M., Oguchi, C.T. Adsorption of 133Cs and 87Sr on pumice tuff: A comparative study between powder and intact solid phase. Acta Geochim 36, 224–231 (2017). https://doi.org/10.1007/s11631-016-0133-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-016-0133-3