Abstract
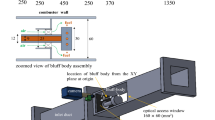
The laminar premixed n-propylamine (NPA) flame with equivalence ratio of 1.70 has been investigated at 4666.28 Pa using tunable synchrotron photoionization and molecular-beam mass spectrometry techniques. Chemical structures and mole fractions of 40 species were determined. Ethenol, allylamine, butadiyne, vinylacetylene, 1,3-butadiene, 1-butene, 2-butene, n-butyl radical, 1,3-cyclopentadiene, cyclopentene, 2-pentene, benzene, toluene, ethylbenzene, 2-propen-1-imine, cyclopropanimine, pyrrole, 2-butenenitrile and n-butylamine were newly identified in the amine flames. Mole fraction profiles of some species including reactants, intermediates and products in the NPA flame were given. HCN and N2 were observed as the primary N-containing products in the NPA flame, which was different from the result that NO was the major N-containing products in previous studies of nitrogen flames. The bond energies of NPA were calculated through quantum chemistry calculations on the basis of density functional theory at the CBS-QB3 level. It showed that the CH3CH2-CH2NH2 bond was the weakest and NPA mainly decomposed to CH2NH2 and C2H5 radicals. The H-abstractions at Cα by OH/O (NPA+OH=CH3CH2CHNH2+H2O and NPA+O=CH3CH2CHNH2+OH) had significant promoting effects on NPA consumption. The N conversion chain of NPA under flame conditions was proposed and detailed analysis with respect to intermediates especially the nitrogen-containing species were provided. The results will enrich the understanding of NPA flame and are essential to further establish the kinetic mechanism.
Similar content being viewed by others
References
Wu L.N., Tian Z.Y., Weng J.J., Yu D., Liu Y.X., Tian D.X., Cao C.C., Zou J.B., Zhang Y., Yang J.Z., Experimental and kinetic study on the low-temperature oxidation of pyridine as a representative of fuel-N compounds. Combustion and Flame, 2019, 202: 394–404.
Zhang H., Liu J., Shen J., Jiang X., Thermodynamic and kinetic evaluation of the reaction between NO (nitric oxide) and char (N) (char bound nitrogen) in coal combustion. Energy, 2015, 82: 312–321.
Glarborg P., Jensen A.D., Johnsson J.E., Fuel nitrogen conversion in solid fuel fired systems. Progress in Energy and Combustion Science, 2003, 29(2): 89–113.
Wojtowicz M.A., Pels J.R., Moulijn J.A., The fate of nitrogen functionalities in coal during pyrolysis and combustion. Fuel, 1995, 74(4): 507–516.
Tian Z.Y., Zhang L.D., Li Y.Y., Yuan T., Qi F., An experimental and kinetic modeling study of a premixed nitromethane flame at low pressure. Proceedings of the Combustion Institute, 2009, 32(1): 311–318.
Brackmann C., Nauclér J.D., El-Busaidy S., Hosseinnia A., Bengtsson P.-E., Konnov A.A., Nilsson E.J.K., Experimental studies of nitromethane flames and evaluation of kinetic mechanisms. Combustion and Flame, 2018, 190: 327–336.
Okafor E.C., Naito Y., Colson S., Ichikawa A., Kudo T., Hayakawa A., Kobayashi H., Experimental and numerical study of the laminar burning velocity of CH4-NH3-air premixed flames. Combustion and Flame, 2018, 187: 185–198.
Liu S., Zou C., Song Y., Cheng S., Lin Q., Experimental and numerical study of laminar flame speeds of CH4/NH3 mixtures under oxy-fuel combustion. Energy, 2019, 175: 250–258.
Ichikawa A., Naito Y., Hayakawa A., Kudo T., Kobayashi H., Burning velocity and flame structure of CH4/NH3/air turbulent premixed flames at high pressure. International Journal of Hydrogen Energy, 2019, 44(13): 6991–6999.
Tian Z.Y., Li Y.Y., Zhang L.D., Glarborg P., Qi F., An experimental and kinetic modeling study of premixed NH3/CH4/O2/Ar flames at low pressure. Combustion and Flame, 2009, 156: 1413–1426.
Hemeryck A., Motta A., Lacaze-Dufaure C., Costa D., Marcus P., DFT-D study of adsorption of diaminoethane and propylamine molecules on anatase (101) TiO2 surface. Applied Surface Science, 2017, 426: 107–115.
Liu Z.J., Atakan B., Kohse-Höinghaus K., Deposition of hexagonal GaN using n-propylamine as a nitrogen precursor. Journal of Crystal Growth, 2000, 219(1): 176–179.
Kim B.K., Ryu S.K., Kim B.J., Park S.J., Adsorption behavior of propylamine on activated carbon fiber surfaces as induced by oxygen functional complexes. Journal of Colloid and Interface Science, 2006, 302(2): 695–697.
Badari A.C., Harnos S., Lónyi F., Onyestyák G., Štolcová M., Kaszonyi A., Valyon J., A study of the selective catalytic hydroconversion of biomass-derived pyrolysis or fermentation liquids using propylamine and acetic acid as model reactants. Catalysis Communications, 2015, 58: 1–5.
Higashihara T., Gardiner W.C., Hwang S.M., Shock tube and modeling study of methylamine thermal decomposition. Journal of Physical Chemistry, 1987, 91(7): 1900–1905.
Kantak M.V., Manrique K.S.D., Aglave R.H., Hesketh R.P., Methylamine oxidation in a flow reactor: Mechanism and modeling. Combustion and Flame, 1997, 108: 235–265.
Li S., Davidson D.F., Hanson R.K., Shock tube study of ethylamine pyrolysis and oxidation. Combustion and Flame, 2014, 161: 2512–2518.
Lucassen A., Zhang K., Warkentin J., Moshammer K., Glarborg P., Marshall P., Kohse-Höinghaus K., Fuel-nitrogen conversion in the combustion of small amines using dimethylamine and ethylamine as biomass-related model fuels. Combustion and Flame, 2012, 159: 2254–2279.
Pappijn C.R., Vin N., Vermeire F.H., Van De Vijver R., Herbinet O., Battin-Leclerc F., Reyniers M.F., Marin G.B., Van Geem K.M., Experimental and kinetic modeling study of the pyrolysis and oxidation of diethylamine. Fuel, 2020, 275(1): 117744.
Garner S., Dubois T., Togbe C., Chaumeix N., Dagaut P., Brezinsky K., Biologically derived diesel fuel and NO formation Part 2: Model development and extended validation. Combustion and Flame, 2011, 158: 2302–2313.
Taylor H., Austin H.E., The thermal decomposition of propylamine. The Journal of Physical Chemistry, 2002, 35: 2658–2666.
Sickman D.V., Rice O.K., The thermal decomposition of propylamine. Journal of the American Chemical Society, 1935, 57(1): 22–24.
Almatarneh M.H., Elayan I.A., Al-Sulaibi M., Al Khawaldeh A., Saber S.O.W., Al-Qaralleh M., Altarawneh M., Unimolecular decomposition reactions of propylamine and protonated propylamine. ACS Omega, 2019, 4(2): 3306–3313.
Verhaak M., Vandillen A.J., Geus J.W., Disproportionation of n-propylamine on supported nickel catalysts. Applied Catalysis a-General, 1994, 109(2): 263–275.
Badari A.C., Harnos S., Lonyi F., Onyestyak G., Stolcova M., Kaszonyi A., Valyon J., A study of the selective catalytic hydroconversion of biomass-derived pyrolysis or fermentation liquids using propylamine and acetic acid as model reactants. Catalysis Communications, 2015, 58: 1–5.
Almatarneh M.H., Omari R., Omeir R.A., Khawaldeh A., Afaneh A.T., Sinnokrot M., Akhras A., Marashdeh A., Computational study of the unimolecular and bimolecular decomposition mechanisms of propylamine. Scientific Reports, 2020, 10(1): 11698.
Yang B., Huang C.Q., Yang R., Wei L.X., Wang J., Wang S.S., Shan X.B., Qi F., Zhang Y.W., Sheng L.S., Wang Z.Y., Hao L.Q., Zhou S.K., Vacuum ultraviolet photoionization and photodissociation of pentafluoroethane. Acta Physico-Chimica Sinica, 2005, 21(5): 539–543.
Qi F., Yang R., Yang B., Huang C., Wei L., Wang J., Sheng L., Zhang Y., Isomeric identification of polycyclic aromatic hydrocarbons formed in combustion with tunable vacuum ultraviolet photoionization. Review of Scientific Instruments, 2006, 77(8): 084101.
Cool T.A., Nakajima K., Taatjes C.A., Mcilroy A., Westmoreland P.R., Law M.E., Morel A., Studies of a fuel-rich propane flame with photoionization mass spectrometry. Proceedings of the Combustion Institute, 2005, 30(1): 1681–1688.
Zhou Z., Mass spectrometry, photoionization. Encyclopedia of Analytical Science, 2019, 6: 483–489.
Jing Wang, Bin Yang, Yuyang Li, Zhenyu Tian, Taichang Zhang, Fei Qi, Nakajima K., The tunable VUV single-photon ionization mass spectrometry for the analysis of individual components in gasoline. International Journal of Mass Spectrometry, 2006, 263(1): 30–37.
Chen J.T., Yu D., Li W., Chen W.Y., Song S.B., Xie C., Yang J.Z., Tian Z.Y., Oxidation study of benzaldehyde with synchrotron photoionization and molecular beam mass spectrometry. Combustion and Flame, 2020, 220: 455–467.
Muller C., Michel V., Scacchi G., Come G.M., Thergas-a computer program for the evaluation of thermochemical data of molecules and free radicals in the gas phase. Journal De Chimie Physique Et De Physico-Chimie Biologique, 1995, 92: 1154–1178.
Photonionization Cross Section Database (Version 2.0), National Synchrotron Radiation Laboratory, Hefei, China, 2017 http://flame.nsrl.ustc.edu.cn/database/.
Cool T.A., Wang J., Nakajima K., Taatjes C.A., Mcllroy A., Photoionization cross sections for reaction intermediates in hydrocarbon combustion. International Journal of Mass Spectrometry, 2005, 247(1): 18–27.
Hansen N., Cool T.A., Westmoreland P.R., Kohse-Höinghaus K., Recent contributions of flame-sampling molecular-beam mass spectrometry to a fundamental understanding of combustion chemistry. Progress in Energy and Combustion Science, 2009, 35(2): 168–191.
Hartlieb A., Atakan B., Kohse-Hoinghaus K., Effects of a sampling quartz nozzle on the flame structure of a fuel-rich low-pressure propene flame. Combustion and Flame, 2000, 121: 610–624.
Struckmeier B.U., Oßwald P., Kasper T., Böhlin L., Heusing M., Köhler M., Andreas B., Kohse-Höinghaus K., Sampling probe influences on temperature and species concentrations in molecular beam mass spectroscopic investigations of flat premixed low pressure flames. International Journal of Research in Physical Chemistry and Chemical Physics, 2009, 223(4): 503–537.
Jin K.R., Tian Z.Y., Effect of thermal radiation heat transfer on the temperature measurement by thermocouple in premixed laminar flames. Journal of Thermal Science, 2021, (Accepted).
Liu M., Jiang X., Fang Y., Guo M., Ding C., Numerical investigation on convective heat transfer of supercritical carbon dioxide in a mini tube considering entrance effect. Journal of Thermal Science, 2021, 30(6): 1986–2001.
Pappijn C.a.R., Vin N., Vermeire F.H., Van De Vijver R., Herbinet O., Battin-Leclerc F., Reyniers M.F., Marin G.B., Van Geem K.M., Experimental and kinetic modeling study of the pyrolysis and oxidation of diethylamine. Fuel, 2020, 275(1): 117744.
Tian D.X., Liu Y.X., Wang B.Y., Cao C.C., Liu Z.K., Zhai Y.T., Zhang Y., Yang J.Z., Tian Z.Y., Pyrolysis study of iso-propylbenzene with photoionization and molecular beam mass spectrometry. Combustion and Flame, 2019, 209(2019): 313–321.
Wang B.Y., Liu Y.X., Weng J.J., Glarborg P., Tian Z.Y., New insights in the low-temperature oxidation of acetylene. Proceedings of the Combustion Institute, 2017, 36(1): 355–363.
Maclean D.I., Wagner H.G., The structure of the reaction zones of ammonia oxygen and hydrazine decomposition flames. Proceedings of the Combustion Institute, 1967, 11(1): 871–878.
Glarborg P., Miller J.A., Ruscic B., Klippenstein S.J., Modeling nitrogen chemistry in combustion. Progress in Energy and Combustion Science, 2018, 67(C): 31–68.
Lucassen A., Labbe N., Westmoreland P.R., Kohse-Höinghaus K., Combustion chemistry and fuel-nitrogen conversion in a laminar premixed flame of morpholine as a model biofuel. Combustion and Flame, 2011, 158: 1647–1666.
Taatjes C.A., Hansen N., Miller J.A., Cool T.A., Wang J., Westmoreland P.R., Law M.E., Kasper T., Kohse-Höinghaus K., Combustion chemistry of enols: Possible ethenol precursors in flames. Journal of Physical Chemistry A, 2006, 110(9): 3254–3260.
Taatjes C.A., Hansen N., Mcilroy A., Miller J.A., Senosiain J.P., Klippenstein S.J., Qi F., Sheng L.S., Zhang Y.W., Cool T.A., Wang J., Westmoreland P.R., Law M.E., Kasper T., Kohse-Höinghaus K., Enols are common intermediates in hydrocarbon oxidation. Science, 2005, 308(5730): 1887–1889.
Hansen N., Miller J.A., Westmoreland P.R., Kasper T., Kohse-Höinghaus K., Wang J., Cool T.A., Isomer-specific combustion chemistry in allene and propyne flames. Combustion and Flame, 2009, 156: 2153–2164.
Man X., Tang C., Zhang J., Zhang Y., Pan L., Huang Z., Law C.K., An experimental and kinetic modeling study of n-propanol and i-propanol ignition at high temperatures. Combustion and Flame, 2014, 161: 644–656.
Tian Z.Y., Li Y.Y., Zhang T.C., Zhu A.G., Cui Z.F., Qi F., An experimental study of low-pressure premixed pyrrole/oxygen/argon flames with tunable synchrotron photoionization. Combustion and Flame, 2007, 151: 347–365.
Memon H.U.R., Bartle K.D., Taylor J.M., Williams A., The shock tube pyrolysis of pyridine. International Journal of Energy Research, 2000, 24(13): 1141–1159.
Mackie J.C., Colket M.B., Nelson P.F., Shock tube pyrolysis of pyridine. Journal of Physical Chemistry, 1990, 94(10): 4099–4106.
Hong X., Zhang L., Zhang T., Qi F., An experimental and theoretical study of pyrrole pyrolysis with tunable synchrotron VUV photoionization and molecular-beam mass spectrometry. Journal of Physical Chemistry A, 2009, 113(18): 5397–5405.
Mcenally C.S., Pfefferle L.D., The effects of dimethyl ether and ethanol on benzene and soot formation in ethylene nonpremixed flames. Proceedings of the Combustion Institute, 2007, 31(1): 603–610.
Riedl Z., Hajos G., Pelaez W.J., Gafarova I.T., Moyano E.L., Yranzo G.I., Flash vacuum pyrolysis (FVP) of 1,2,4-benzotriazine derivatives. Tetrahedron, 2003, 59(6): 851–856.
Zhao, L.; Xie, M.; Ye, L.; Cheng, Z.; Cai, J.; Li, Y.; Qi, F.; Zhang, L., An experimental and modeling study of methyl propanoate pyrolysis at low pressure. Combustion and Flame, 2013, 160: 1958–1966.
Alessio F., Alberto C., Tiziano F., Ulrich N., Eliseo R., Reinhard S., Kalyanasundaram S., An experimental and kinetic modeling study of n-propanol and iso-propanol combustion. Combustion and Flame, 2010, 157: 2–16.
Li W., Zhang Y., Mei B., Li Y., Cao C., Zou J., Yang J., Cheng Z., Experimental and kinetic modeling study of n-propanol and i-propanol combustion: Flow reactor pyrolysis and laminar flame propagation. Combustion and Flame, 2019, 207: 171–185.
Acknowledgment
The authors are grateful for the funding supports from National Natural Science Foundation of China (No. 52161145105), the Ministry of Science and Technology of China (No.2017YFA0402800), Beijing Municipal Natural Science Foundation (JQ20017), K.C. Wong Education Foundation and Recruitment Program of Global Youth Experts. The authors also thank the researchers in NSRL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Chen, J., Yang, J. et al. Investigation of the Laminar Premixed n-Propylamine Flame. J. Therm. Sci. 31, 854–866 (2022). https://doi.org/10.1007/s11630-022-1528-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11630-022-1528-6