Abstract
To optimize protocol for obtaining hairy roots of Nitraria schoberi L. with high antiviral activities, factors such as four strain types of Agrobacterium rhizogenes (A4, ATCC15834, R-1601, 8196), two explant types, namely cotyledonous and primary leaves of seedlings, and different cultivation durations (30 and 90 d) were studied. The formation of hairy roots was observed after 2 to 4 wk of incubation, depending on the type of explant and the strain of A. rhizogenes used. The maximum transformation frequency (85.7%) was observed in the cotyledons genetically modified with the strain ATCC15834. The transgenic nature of hairy roots was revealed by PCR with primers to the Agrobacterium oncogenes rolB and rolC. The absence of contamination of the culture by A. rhizogenes was confirmed by primers to the virC and virD1 genes. Phytochemical analysis showed that accumulation of individual metabolites in the line samples exceeded their levels in the native Nitraria roots. Catechin content in the cultures of long-term cultivation (90 d) was found 1.4 to 2.2 times higher than the same samples of short cultivation (30 d) and 4.8 to 10.8 times higher in comparison with the native roots. The most productive in terms of catechin level were hairy roots of long-term cultivation obtained during the transformation of primary leaves of N. schoberi seedlings with ATCC15834 strain. These data were consistent with the highest antiviral activities against influenza viruses of A (H5N1) and A (H3N2) subtypes with neutralization indexes 6.5 to 6.75 log10, and selectivity index values were in the range 15.4 to 16.4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Influenza is an acute respiratory illness that can progress to more severe consequences, including secondary pneumonia and other bacterial infections, cardiovascular events, asthma attacks, neurologic complications, and social impacts (Macias et al. 2021). It is a highly contagious infection that has a devastating effect on a global scale, leading to numerous deaths. The damage of respiratory ciliated cells due to influenza can also facilitate the conditions for infection with SARS-CoV-2. A recent study has illustrated that seasonal influenza has a unique ability to enhance SARS-CoV-2 infection, and thus, prevention of influenza is of great significance during the COVID-19 pandemic (Bai et al. 2021).
Species of the genus Nitraria L. (saltpeter), halophytic shrubs of the family Nitrariaceae that can survive under extreme drought and in saline-alkali environments, are promising objects for the development of novel therapeutic agents. Due to the important mechanism of stress resistance to severe growing conditions, members of this genus accumulate valuable metabolites, including polyunsaturated fatty acids, phenolic acids, phytosterols, flavonoids, and alkaloids, of which flavonoids and alkaloids are the most abundant compounds (Tulyaganov and Allaberdiev 2003). It is known that N. schoberi fruit, leaf, and root extracts possess strong antioxidant properties and are also characterized by fungicidal, antimicrobial, and antiinflammatory activities (Sharifi-Rad et al. 2015). The cytotoxic effect of N. retusa leaf extracts on melanoma cells was established (Boubaker et al. 2015), as well as their antiviral activity against Wa rotavirus, adenovirus subtype 7, and Coxsackie B4 virus (Mohamed et al. 2015). Additionally, for the first time, the results of our previous study demonstrated that N. schoberi hairy roots (HRs) possessed high antiviral activity against influenza virus subtypes A(H5N1) and A(H3N2) (Zheleznichenko et al. 2018). Moreover, extracts from berries of N. tangutorum significantly reduced the infectivity of the human influenza A/H3N2 virus in vitro in Madin-Darby canine kidney (MDCK) cell culture (Kurskaya et al. 2022).
Species of the genus Nitraria can be considered sources of valuable metabolites for the production of novel antiviral drugs. However, the raw material collection of Nitraria for medical purposes might inflict serious damages on natural populations. N. schoberi is known to be endangered species (Paunescu 2011) due to its long generative cycle, seed dormancy, low genetic diversity, and sensitivity to some biotic and abiotic stresses (Bahadori and Javanbakht 2006).
An attractive solution to the problem of raw material shortage is the use of alternative biotechnological approaches through the establishment of a culture of genetically modified roots or HRs by A. rhizogenes. HRs possess an increased ability to synthesize secondary metabolites on nutrient media without plant growth regulators (Tian 2015). In addition, the characteristic peculiarities of HRs are rapid growth, frequent branching, genetic stability, and rapid mass reproduction (El-Esawi et al. 2017).
In the first report, the high antiinfluenza activity of HR lines, obtained by transforming the seedlings’ primary leaves with a wild strain of A. rhizogenes ATCC 15,834, was shown by Zhelesnichenko et al. (2018). At present time, there is no other data on lines of HRs of other Nitraria species and on their antiviral activity. Further research is needed to obtain more detailed information on the antiviral activity of HR species of the genus Nitraria and, in particular, N. schoberi.
The aims of the present study were as follows: (i) to initiate the formation of N. schoberi HR lines from different types of explants using the fourth A. rhizogenes strains (A4, ATCC15834, R-1601, 8196 RT), (ii) by varying the time of the cultivation to assess the productivity of HR lines in terms of the secondary metabolites; and (iii) to evaluate the antiviral activity of their extracts against influenza A (H5N1) and A (H3N2).
Materials and methods
Plant material and obtaining of seedling
N. schoberi seeds were collected in the Blagoveshchensk District of the Altai Region (coordinates 52° 52′ 94″ N, 79° 71′ 60″ E) in 2019. The seeds have been collected from three different plants in excess quantities from each individual plant. The seeds were scarified and sterilized as described in a previous study (Zheleznichenko et al. 2016). Embryos were isolated from sterile seeds and germinated on the solid medium of Murashige and Skoog (MS; Murashige and Skoog 1962) without plant growth regulators (PGRs) at a temperature of 23 ± 2°C, 16/8 h (light/dark) photoperiod, and illumination at 60 µmol m−2 s−1.
Preparation of Agrobacterium and transformation procedure
Wild (unmodified) agropine strains of Agrobacterium specifically A4, ATCC15834, R-1601, and mannopine 8196 RT (Kuluev et al. 2015) were used in the experiments and maintained at 4°C. Before the transformation, Agrobacterium was inoculated on a solid YEB medium (Vervliet et al. 1975) and cultivated in a thermostat (at 25 ± 2°C) shielded from light for 48 h. Then, Agrobacterium cultures were scraped off the surface of the solid YEB medium with a Drigal spatula and resuspended in a liquid MS medium. The detailed protocol for inducing HR cultures was described earlier by Zheleznichenko et al. (2018). Briefly, the explants (cotyledons and primary leaves of aseptic seedlings) were cut with a scalpel and were immersed overnight in Agrobacterium suspension with an optical density OD600 = 0.4. After that, the plant material was washed three times with sterile distilled water and dried on sterile filter paper. To eliminate bacteria, the explants were cultured on a solid medium Dunstan and Short (BDS; Dunstan and Short 1977) without PGRs containing 500.0 mg L−1 cefotaxime (Duchefa Biochemie, Netherlands) for 1 mo. HRs formed were separated from the explants, transferred and incubated to a nutrient medium of the same composition, and cultivated for several passages.
Cultivation of HR cultures
Well-grown HRs were transferred into a hormone-free liquid BDS nutrient medium without antibiotics and cultured in flasks on a shaker (Elmi, S-3–02 L, Latvia) at 100 rpm and temperature 22 ± 1°C in the dark. The duration of the passage was 30 d. The HR culture of the fourth passage was used for further analysis, and the duration of incubation differed and was either 30 or 90 d (Table 1). During the long-term incubation (90 d), 30 mL of the fresh nutrient medium was added to the flasks every 30 d. Three flasks were used for each sample, and the experiments were repeated three times. After cultivation, HRs were washed with distilled water and dried at room temperature on filter paper. The dried plant material was used for molecular and phytochemical analysis. The cytotoxicity and antiviral activity of extracts of the obtained roots were also evaluated. For comparison, the roots of a 5-yr-old N. schoberi plant introduced into the experimental plot were used.
PCR analysis for the rol and vir genes
DNA from HRs and roots of native plants was isolated according to the protocol of the DiamondDNA Plant Kit D commercial kit (Diamond DNA, Barnaul, Russia). Before PCR, all DNA samples were diluted to 5.0 ng µL−1 with DNA dilution buffer (Evrogen, Moscow, Russia). DNA from untransformed N. schoberi roots served as a negative control. As a positive control, plasmid DNA of the studied A. rhizogenes strains was used, which was isolated using the Plasmid Miniprep kit (Evrogen, Moscow, Russia). Putative transgenic roots (HRs) were tested for the presence of the rolB (780 bp) (Sharan et al. 2019) and rolC (543 bp) (Thiruvengadam et al. 2014) genes. PCR amplification was performed using primers for the rolB gene (forward 5′-ATGGATCCCAAATTGCTATTCCTTCCACGA-3′ and reverse 5′-TTAGGCTTCTTTCTTCAGGTTTACTGCAGC-3′). The primers for rolC gene were upper 5′-ATGGCTGAAGACGACCTGTGTT-3′ and lower 5′-TTAGCCGATTGCAAACTTGCAC-3′. Moreover, PCR analysis with the virC (730 bp) (Sawada et al. 1995) and virD1 (441 bp) (Vinterhalter et al. 2015) was performed to refute any bacterial contamination. The following primer pairs were used to identify the virC gene: sense 5′-ATCATTTGTAGCGACT-3′ and antisense 5′- AGCTCAAACCTGCTTC-3′ and to detect virD1: forward 5′-ATGTCGCAAGGACGTAAGCCCA-3′ and reverse 5′-GGAGTCTTTCAGCATTGGAGCAA-3′. PCR was performed in a C-1000 thermal cycler (Bio-Rad, Hercules, CA). Amplification was carried out as described earlier by Zheleznichenko et al. (2018). Amplification products were stained with 3 × SYBR-Green (Medigen, Novosibirsk, Russia), then separated in 1.7% agarose gel in 1 × TAE buffer at a voltage of 2.7 V cm−1. Gels were considered and documented using the Gel Doc XR + system (Bio-Rad). The size of the amplified fragments was determined using a 100 bp DNA marker (Evrogen, Moscow, Russia).
Extraction procedure
The phytochemical composition of the 4th passage of HR culture and native roots of N. schoberi were studied in ethanol extracts. Antiviral activity was evaluated in both ethanol and aqueous extracts. Ethanol extracts were prepared from crushed homogenized dry raw material of HR culture and from dried biomass of native roots, which were suspended three times in 30 mL of 70% aqueous ethanol solution for 8 h at 60°C. The combined cooled extracts were then filtered through glass filters, evaporated on a rotary evaporator, and dried at 60°C in a desiccator. Aqueous extracts were prepared from the raw material by its extraction in 60 mL of purified water at 95°C for 1 h. Then, the extract was separated from the raw material through a glass filter and the extraction procedure was repeated with 40 mL of water. The cooled extracts were evaporated and dried at 60°C.
Determination of flavonoid
According to the previously described protocols, flavonoid quantity was assayed (Zheleznichenko et al. 2018). Flavonoid content was analyzed using the complexation reaction of flavonoids with AlCl3, according to Brighente et al. (2007). Rutin (Sigma-Aldrich, St. Louis, MO) was used to construct a calibration curve. Data were expressed as mg rutin equivalents g−1 dry weight of the sample (mg RE g−1).
Phenolic acid content
The total content of phenolic (hydroxycinnamic) acid was determined according to the method of Matkowski et al. (2008) using Anrow reagent (1.0 mL of 10.0% w/v of sodium molybdate and 10.0% w/v of sodium nitrite) and NaOH (1.0 mL, 1.0 M) and filled up to 10.0 mL. Thereafter, absorbance was measured at 490 nm using an SF-26 spectrometer (LOMO, St. Petersburg, Russia). The results were expressed as mg of caffeic acid equivalents per gram dry weight of the sample (mg CAE/g).
Determination of pectin
The amount of pectin was assessed as described in a previous study (Zheleznichenko et al. 2018) using the carbazole method, based on obtaining a specific violet-pink staining of uronic acids with carbazole in a sulfuric medium (McComb and McCready 1952). The amount of pectin was determined from the calibration curve for galacturonic acid. The content of water-soluble pectin or protopectin was defined as mg of galacturonic acid equivalents g−1 dry weight of the sample (mg GAE g−1).
Saponin assay
The content of saponins was determined by direct spectrophotometry in terms of oleanolic acid (Pisarev et al. 2009). A sample of air-dry raw material was extracted three times with 96.0% ethyl alcohol in a boiling water bath. A 10.0 mL aliquot was taken from the combined extraction and evaporated to dryness. The residue was dissolved in a 10 mL hydrolysis mixture (glacial acetic acid-hydrochloric acid–water 3.5:1:5.5) and heated in a water bath for 2 h. Then, the mixture was diluted with water by a factor of 2; the precipitate was filtered off, washed with water, and dissolved in 25.0 mL of hot ethyl alcohol. To 1.0 mL of the obtained solution, 4.0 mL of concentrated sulfuric acid was added and incubated for 10 min, and the optical density was measured at λ = 310 on an SF-56 spectrometer (LOMO, St. Petersburg, Russia), the reference solution being concentrated sulfuric acid. The total content of saponins in the samples was determined in terms of percent oleanolic acid.
HPLC assay
The content and composition of catechins were studied on an analytical HPLC system consisting of a chromatograph Agilent 1200 with a diode array detector and a ChemStation system according to the protocol described in our previous study (Zheleznichenko et al. 2018). The individual compound quantitative estimations in the eluates were quantified according to an external standard method (Van Beek 2002). Standard samples (Sigma-Aldrich) were prepared at 10.0 μg mL−1 concentration in ethyl alcohol. UF spectra of unidentified components were specific for catechins: the spectral range was 246 to 325 nm with λ max 270 to 280 nm. The amount of catechins was expressed as milligram of ± catechin equivalents per gram dry weight of the sample (mg CE g−1).
Assay of cytotoxicity and antiviral activity
To test the cytotoxicity and antiviral activity of the extracts, we used a transplanted MDCK cell culture obtained from the cell culture collection of the Federal Budgetary Institution of Science “State Scientific Center for Virology and Biotechnology “Vector” of the Federal Service for Supervision of Consumer Rights Protection and Human Welfare.” MDCK cells were grown in 96-well plates in an RPMI-1640 medium (Biolot, St. Petersburg, Russia) containing 10.0% inactivated fetal calf serum (FCS) (HyClone, Logan, UT) and antibiotics (penicillin 100 IU mL−1, streptomycin 100 µg mL−1, Rapharma, Moscow, Russia). The plates with cells were placed in an incubator at + 37°C, 5.0% CO2, and 100% humidity for 2 to 3 d until a cell monolayer was formed.
Toxic concentrations were determined by diluting sample extracts with the RPMI-1640 medium 2, 4, 8, 20, 40, 200, and 400 times. The evaluation of the toxic effect in the monolayer of the MDCK cell culture was performed using an inverted microscope. For this purpose, 10.0 mg of each sample extract was dissolved in 5.0 mL of the RPMI-1640 medium. The dissolution was performed using a shaker at ca. 40°C. Then, the sample solutions were diluted, 100.0 µL was put in the corresponding wells of the plates, and the plates were placed in a thermostat at + 37°C, 5.0% CO2, and 100% humidity. The presence of toxic effects in MDCK cell culture and the maximum tolerated concentration (MTC) at which 100% of the cells remained in the monolayer were assessed after 2 d.
Antiviral assay of drugs against influenza A virus
In experiments to test the antiviral activity of plant extracts, influenza A virus strains were used: the strain of avian influenza virus A/chicken/Kurgan/05/2005 (H5N1) and human influenza virus A/Aichi/2/68 (H3N2) adapted to laboratory mice, obtained from the State collection of viral infections and rickettsiosis agents of the State Research Center VB “Vector.”
The antiviral efficacy of N. schoberi extracts was estimated by the change of infectivity (titer) of influenza A/Aichi/2/68 (H3N2) and A/chicken/Kurgan/05/2005 (H5N1) virus in MDCK cell monolayer when the maximum tolerated concentration (MAC) of tested samples for given cell culture was used. For this purpose, 50.0 μL of each extract diluted with RPMI-1640 medium containing 2.0 μg mL−1 TPCK trypsin (Sigma-Aldrich) and 50.0 μL of tenfold dilutions of the virus-containing liquid (1st to 8th) prepared in the same medium were added to the cell culture monolayer. Experiments were performed in 4 replicates. Cells were incubated for two d at 37°C in a 5.0% CO2 atmosphere. After 2 d in each well, the presence of the virus in the culture medium was registered by hemagglutination reaction with 1% suspension of chicken erythrocytes and expressed as log10 TCD50 mL−1. Tamiflu (La Roche Ltd., Switzerland) at a concentration of 0.05 mg mL−1 was used as a reference drug. In the virus control, 50.0 μL of the nutrient medium was used instead of the drugs. The virus inhibitory effect of the studied extracts was estimated by the decrease in virus titer in the experiment compared to the control; for this purpose, the neutralization index (IN) of viruses under the influence of the preparations was calculated: IN = virus titer in the control − virus titer in the experiment (log10).
Colorimetric method for determining the cytotoxicity and antiviral activity of samples in vitro
Evaluation of the cytotoxicity and antiviral efficacy was performed using colorimetry. The optical density (OD) of a dye solution absorbed by living cells in a monolayer was measured (Smee et al. 2002). Dry extracts of the samples of N. schoberi were dissolved in a supporting medium DMEM containing 2.0 µg mL−1 of tripsine TPCK (Sigma-Aldrich) and analyzed in over the concentration range 61.7 to 5000.0 µg mL−1. The reference drug Tamiflu was studied over the concentration range of 0.046 to 100.0 µg mL−1.
The toxicity of the samples was evaluated as follows. To study the toxicity of the drugs, 0.1 mL of the sample at a concentration of 5.0 mg mL−1 and 0.1 mL of its 4 consecutive dilutions with a step of 3 were added to the wells of 96-well plates with MDCK cells with 0.1 mL of DMEM maintenance medium. The plates were then incubated for 2 d at 37°C and 5.0% CO2. The study of the antiviral activity of the samples was carried out according to the “treatment and prophylactic procedure,” as described below. In this procedure, 0.1 mL of a sample at a concentration of 10.0 mg mL−1 and 0.1 mL of its 4 consecutive dilutions with a step of 2 were added to the wells of 96-well plates with MDCK cells; then, the cells were incubated at 37°C and 5.0% CO2. After 2 h, the monolayer was infected with a virus-containing liquid at a dose of 100 TCID50 containing 2.0 μg mL−1 trypsin in a volume of 0.1 mL. To the wells containing the virus for control, 0.1 mL of DMEM support medium was added instead of the samples studied.
After 3 d of incubation in an atmosphere of 5.0% CO2 at 37°C, the culture medium was removed and vital dye MTT was added to each well of the plate in a volume of 0.75 mL with a concentration of 1.0 mg mL−1, dissolved in Dulbecco’s phosphate-buffered saline (DPBS). The plates were incubated for 90 min at 37°C; then, the supernatant was removed and 0.1 mL of DMSO was added. After 10 min, OD was measured on a multifunctional spectrophotometer iMark (Bio-Rad) in 96-well plates at a wavelength of 540 nm, which is an indicator of the number of viable cells in a monolayer preserved under the cytopathic effect of the virus or the cytotoxic effect of the sample.
The results of measuring OD depending on the concentration of the drug were presented in a semi-logarithmic coordinate system and the 50.0% toxic concentration (CC50 in µg mL−1) and 50.0% inhibitory (effective) concentration (IC50 in µg mL−1) of the drug were calculated using a computer program SoftMaxPro-4.0. CC50 is the concentration of a substance in a nutrient medium at which 50.0% of cells in an uninfected monolayer are destroyed (loose viability). IC50 is the concentration of a substance in the nutrient medium at which 50% of the cells in the infected monolayer are not destroyed (remain viable). Based on these values, the selectivity index (SI) of the test sample was calculated: SI = CC50 µg/mL/IC50 µg mL−1. Connections with SI above 8 are considered active (Mironov 2012).
Statistical analysis of data
Statistical processing of the obtained results and their comparison were performed using standard methods and Statistica 6.0 software package (Rebrova 2006). When processing the results of in vitro experiments, the experimental and control VH titers were calculated and expressed in decimal logarithms of 50% tissue cytopathic doses in mL (log10 TCD50 mL−1), presented as M ± SM (where M is the mean value, SM is the standard deviation) and compared by the Spearman-Kerber method (Mahy and Kangro 1996). Differences were considered reliable at a 95% level of reliability (p ≤ 0.05).
Results and discussion
Effect of A. rhizogenes strains and the type of explants on HR initiation
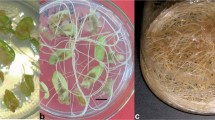
The transformation of different N. schoberi explants by A. rhizogenes strains studied was successful (Fig. 1a). The formation of genetically modified roots was noted after 2 to 4 wk of incubation, depending on the type of the explant and the strain of Agrobacterium used. The most virulent of Agrobacterium strains studied was found to be strain R-1601. During the transformation of both cotyledons and primary leaves, over 90% of the explant formed HRs. Also, during the transformation with this strain, the maximum number of genetically modified roots per explant was noted in comparison with the studied strains (Table 2). However, further cultivation of the roots failed to eliminate Agrobacterium after ceftriaxone treatment; therefore, the growth and the development were practically absent; HRs were getting brown and, finally, died. The transformation frequency of the cotyledon leaf explant by strain 8196 RT (HR8196-C) and ATCC15834 (HR15834-C-30, HR15834-C-90) was more than 80%. The A4 strain showed a lower virulence; the transformation frequency was about 70% (HRA4-C). The transformation efficiency of primary leaves by Agrobacterium strains used was less than cotyledon. For example, the transformation of primary leaves with strain ATCC15834 (HR15834-L-30, HR15834-L-90) induced the formation of HRs in more than 70% of explants. Whereas the transformation frequency with strain 8196 RT (HR8196-L-30, HR8196-L-90) and strain A4 (HRA4-L-30, HR A4-L-90) was less efficient.
The number of roots per the explant during the transformation with strains 8196 RT and ATCC15834 was lower compared with strain R-1601, and the least effective strain was A4. During the transformation of cotyledon leaves, the number of roots per explant was slightly higher than that of primary leaves; however, it depended more on the strain used than on the explant.
Thus, it was noted that all A. rhizogenes strains showed virulence against N. schoberi. The most effective for the transformation of the explant studied was the agropine strain ATCC15834, while the transformation of the cotyledon leaves was more active than the primary leaves. Although agropine strains of Agrobacterium are considered to be more virulent (Lee et al. 2010), the strain A4 in the current study showed low activity during the transformation of N. schoberi. ATCC15834 strain was widely used for genetic transformation in many plants such as Astragalus membranaceus (Fisch. Ex Link) Bunge, (Novikova et al. 2020), Astragalus penduliflorus Lam (Ambros et al. 2020; Novikova et al. 2022), Gentiana dinarica Beck. (Vinterhalter et al. 2015), and Polyscias filicifolia (Sliwi´nska et al. 2022). The roots obtained demonstrated plagiotropic growth (Fig. 1c), which is a morphological feature of genetically modified HRs (Tepfer 1984). Well-growing clones were selected and transferred to liquid hormone-free nutrient media (Fig. 1c). The phenotype of genetically modified roots is associated with the expression of rol (root oncogenic loci) genes (A, B, C, and D), which are responsible for their formation (Le Flem-Bonhomme et al. 2004; Hong et al. 2006), and, in particular, the rolB gene (Pavlova et al. 2014). The protein products of these genes increase the susceptibility of plant cells to auxin and cytokinin (Estruch et al. 1991). These four rol genes (A, B, C, and D) are usually present in the TL region of Agrobacterium Ri plasmids. At the same time, agropine strains (ATCC15834, A4) transform into the plant genome independently of each other, both TL-DNA and TR-DNA, while mannopine strains only TL-DNA (Le Flem-Bonhomme et al. 2004; Hong et al. 2006).
Confirmation of HR transgenic status
The molecular evidence of transgenic status obtained HRs was demonstrated by PCR analysis with primers to rol genes in particular of genes rolB and rolC (Fig. 2a, b). Bands of amplified DNA fragments of 780 (rolB) and 543 (rolC) bp from genomic DNA HRs were observed. This was a confirmation of the genetic transformation of the obtained roots. The lack of 730 and 441 bp bands (Fig. 2c, d) corresponding to bacterial virC and virD1 in HRs indicated that the obtained root culture was free of Agrobacterium contamination.
PCR amplification of the rolB (a), rolC (b), and virD1 (c) virC (d) genes of hairy root cultures: lane M—molecular weight marker (100 bp); lane 1—genomic DNA of non-transformed Nitraria schoberi L. (negative control); lane 2—plasmid DNA of Agrobacterium rhizogenes ATCC15834 (positive control); lane 3—plasmid DNA of A. rhizogenes 8196 RT (positive control); lane 4—plasmid DNA of A. rhizogenes A4 (positive control); lane 5—genomic DNA of HR15834-C-30 culture; lane 6—genomic DNA of HR15834-C-90 culture; lane 7—genomic DNA of HR15834-L-30 culture; lane 8—genomic DNA of c HR15834-L-90 culture; lane 9—genomic DNA of HRA4-L-30 culture; lane 10—genomic DNA of HRA4-L-90 culture; lane 11—genomic DNA of HR8196-L-30 culture; lane 12—genomic DNA of HR8196-L-90 culture.
Phytochemical study of HRs
Over the past decade, interest in the antiviral activity of natural compounds has been growing. Recent studies (Kapoor et al. 2017) have established the antiviral properties of various phytochemicals, ranging from flavonoids and lignans to saponins. Another group of researchers published a review of the antiviral effects of six classes of flavonoids (Zakaryan et al. 2017). Also, the antiviral properties of flavonoids against various groups of viruses, ranging from hepatitis to influenza virus, were discussed in detail in the review by Ninfali et al. (2020). Many works are devoted to the study of the antiviral properties of hydroxycinnamic acids (Spasova et al. 2008; Stankova et al. 2009), as well as polysaccharides, including pectins and protopectins (Cheng et al. 2019; Godoi et al. 2019; Kurskaya et al. 2022).
The comparative content of secondary metabolites in the samples of HR culture of the 4th passages of different origins and durations of cultivation and native roots of N. schoberi are shown in Table 3. Flavonoid accumulation in the HR cultures varied depending on the type of the explant from which the lines were obtained, the strain of Agrobacterium used for transformation, and the duration of cultivation.
It was noted that the transformation of both cotyledons (HR15834-C-30, HR15834-C-90) and primary leaves (HR15834-L-30, HR15834-L-90) of Nitraria with A. rhizogenes strain ATCC15834 resulted in 1.6 and 1.1 times decrease in flavonoid content after long cultivation (90 d), respectively. In contrast, when primary leaves were transformed with A. rhizogenes strain 8196 RT (HR8196-L-30, HR8196-L-90), increasing the duration of cultivation contributed to the accumulation of flavonoids 4.8-fold. And when primary leaves were transformed with A. rhizogenes A4 strain (HRA4-L-30, HRA4-L-90), the duration of cultivation had no significant effect on flavonoid accumulation. It was also observed that the flavonoid accumulation level in the cultures of genetically modified roots was 1.2 to 2.0 times higher than in the native roots. The analysis of hydroxycinnamic acids in HR cultures revealed no dependence of their content either on the explant or on the strain of Agrobacterium used for transformation, or on the duration of cultivation. The amount of these compounds varied insignificantly between different samples, and the difference ranged from 4 to 13%. At the same time, hydroxycinnamic acids accumulated 1.2 to 2.3 times more actively in genetically modified roots than in native roots of nitre bushes.
The level of pectins in HR lines obtained by transformation of cotyledons (HR15834-C-30, HR15834-C-90) and primary leaves (HR15834-L-30, HR15834-L-90) with ATCC15834 strain was approximately the same and did not depend on the duration of cultivation. When primary leaves were transformed with Agrobacterium strains 8196 RT and A4, the amount of pectins was decreased approximately by 10% in root cultures of short cultivation (30 d) and by 20% in roots of long cultivation (90 d) than when explants were transformed with strain ATCC15834. At the same time, compared with native roots, the pectin content in HR cultures was 1.5 to 2.4 times lower. As for protopectins, their amount in cultures of transformed roots depended more on the duration of cultivation than on the origin of the culture.
With increasing the duration of cultivation, this index increased by 1.2 to 1.5 times in the samples studied, except for samples HRA4-L-30 and HRA4-L-90, where the content of protopectins, on the contrary, decreased by 2 times. Also, the amount of protopectins in the genetically transformed roots exceeded this level, depending on the sample studied, by 1.2 to 3.2 times compared with the roots of the intact plant. The amount of saponins in the cultures of genetically modified roots depended on the origin of the culture and on the duration of cultivation, but was an order of magnitude lower compared to native roots. The only exception was the samples of long-term cultivation transformed with strain 8196 RT (HR8196-L-90) and A4 (HRA4-L-90) in which the saponin content was two times lower than in the roots of intact plants. Thus, when studying the composition and the content of secondary metabolites in different samples of HR culture, no general pattern of their accumulation was revealed. At the same time, other researchers found that the efficiency of transformation and accumulation of secondary metabolites in HRs is strain-specific and depends on the explants used (Tavassoli and Safipour Afshar 2018; Sathasivam et al. 2022). For example, during the transformation of Althea officinalis, the maximum frequency of HRs formation was observed when using shoot as explants and ATCC15834 strain. Moreover, the highest total content of phenolic and flavonoids was found during the transformation of the same explants with the A4 strain (Tavassoli and Safipour Afshar 2018). For the effective transformation of Ocimum basilicum and the accumulation of the maximum level of rosemary acid, the R-1601 strain was the most effective (Sathasivam et al. 2022). These observations indicate that there is no universal way for obtaining highly productive HRs. On the other hand, for HRs of Catharanthus roseus, it was shown that the production of the secondary metabolites correlates with cultivation duration which is consistent with the current results (Leduc et al. 2006).
Interest in the detailed study of catechins is because their antiinfective properties with different modes of action on different families of viruses have been discovered. For example, catechins and their derivatives can inhibit the activity of herpes simplex virus by direct inhibition of viral particles (Lyu et al. 2005; Isaacs et al. 2008), human immunodeficiency virus (HIV) (Yamaguchi et al. 2002; Williamson et al. 2006), and chikungunya virus (Weber et al. 2015). These compounds have been found to be potent inhibitors of the hepatitis C virus (HCV) and hepatitis B virus (HBV) (Fukazawa et al. 2012). Recent studies have shown that epigallocatechin-3-gallate (EGCG) from green tea extract can suppress coronavirus infections, including SARS-CoV-2 (Henss et al. 2021).
Catechins are also considered to be potential agents against the influenza virus (Kim et al. 2013). In vitro studies have shown that EGCG minimizes influenza A and B virus infectivity in MDCK cell culture (Steinmann et al. 2013). In addition, EGCG and (-)epicatechin gallate (ECG) suppress viral RNA activity, which reduces viral spread (Kuzuhara et al. 2009). Catechins prevent the virus from interacting with the cell membrane when it enters the cell (Saha et al. 2010).
When studying the composition of catechins in extracts of HRs of short (30 d) and long (90 d) cultivation of N. schoberi by HPLC method, 13 compounds of a catechin nature were found (Table 4). Comparison of signal retention times of substances on chromatograms of analyzed samples with retention times of standard samples and spectra allowed us to identify gallic acid (tR 2.7 min), ± -catechin (tR5.4 min), and L-epicatechin (tR 8.6 min) (Fig. 3). It should be noted that the qualitative composition of compounds of catechin nature is more diverse and their quantitative content is significantly higher in long-cultured roots than in the corresponding short-cultured samples. For example, HRs obtained from seedlings transformed with Agrobacterium ATCC15834 strain (HR15834-C-30, HR15834-C-90) during both short (30 d) and long (90 d) cultivation contained 11 compounds of catechin nature but their qualitative composition was different. In the sample of long cultivation (HR15834-C-90), the total content of catechins was 1.7 times higher than in the same sample of short cultivation (HR15834-C-30). In HR lines obtained from primary leaves transformed with the same strain, the short-cultured sample (HR15834-L-30) contained 10 compounds of a catechin nature, while in the long-cultured sample (HR15834-L-90), 14 catechins were found, and their level was 3.8 times higher than that in the short-cultured lines. The same trend was observed in samples obtained from primary leaves but transformed with Agrobacterium 8196 RT strains (HR8196-L-30, HR8196-L-90) and/or A4 (HRA4-L-30, HR A4-L-90). The qualitative composition of catechins was more diverse in long-term cultivation samples, and their total content was also 1.4 and 2.1 times higher than in short cultivation samples, respectively. In the native root of an untransformed plant (control), the number of compounds of catechin nature was 13, which was not significantly different from the genetically modified roots, but their total content was 2.7 to 10.8 times lower than in the samples of HRs. Thus, it can be noted that genetically modified roots are the most productive in terms of catechin accumulation in comparison with native roots of perennial plants, especially in the lines of long-term cultivation.
Since, according to the results of phytochemical analysis, the content of catechins in the genetically modified roots of long-term cultivation was higher than in the same samples of short cultivation, the antiviral activity was studied in ethanol and water extracts of HRs only of long-term cultivation (HR15834-C-90, HR15834-L-90, HRA4-L-90, HR8196-L-90) of the 4th passages.
Cytotoxicity assay and antiinfluenza activities
Before studying the antiviral activity of ethanol and aqueous extracts of N. schoberi, their cytotoxicity was evaluated in vitro (in MDCK cell culture). It was found that the maximum tolerated concentration (MTC) of HRs samples was 1.0 mg mL−1, except for the control aqueous extract from the intact tap root, which showed greater toxicity, and its MPC being 0.25 mg mL−1 (Table 5).
The results of the inhibition study of influenza virus reproduction of strains A/chicken/Kurgan/05/2005 (H5N1) and A/Aichi/2/68 (H3N2) in MDCK cells under the action of extracts showed that both aqueous and ethanolic extracts demonstrate antiviral action against both influenza virus subtypes from all samples of N. schoberi HRs, irrespective of the explant type (cotyledons or primary leaves) and Agrobacterium strain used for the transformation. In the study of the antiviral activity of aqueous extracts of N. tangutorum fruits, high virus inhibitory activity against influenza virus A/H3N2 was shown against the background of low cytotoxicity (Kurskaya et al. 2022). This is consistent with our results. Nevertheless, our research is aimed not only at finding new natural antiinfluenza drugs, but also at developing a method for obtaining standardized medicinal raw materials regardless of the season and without affecting natural plant populations. Simultaneously the extracts obtained from the roots of the intact plant had either the lowest IN values or no virus inhibitory effect at all (IN = 0). Ethanol extract of HRs obtained by transformation of primary leaves of N. schoberi seedlings with Agrobacterium strain ATCC15834 had the highest antiviral activity against both strains of influenza virus (HR15834-L-90). Its neutralization indices reached 6.5 to 6.75 log10. According to the guidelines for the experimental (preclinical) study of new pharmacological substances, a substance has high activity if it reduces the infectivity of viruses in cell culture by 100 and more times (IN ≥ 2.0) (Habriev 2005). In general, the extracts obtained from the culture of N. schoberi HRs reduced the infectivity of the influenza virus by 2.5 to 6.75 log10, so they are promising experimental samples for further study and the creation of drugs based on them with antiinfluenza activity.
The determined parameters CC50 and IC50 of the samples of HRs obtained by prolonged cultivation in comparison with those obtained from the roots of the intact plans by aqueous and ethanolic extractions are summarized in Table 6. The data indicate that all samples obtained using ethanolic extraction have antiviral activity against the influenza A/Aichi/2/68 (H3N2) virus.
The most active was the ethanolic extract of the sample HR15834-L-90, whose IC50 was only 304.0 µg mL−1. Ethanolic extracts from the same samples also exhibit antiviral activity against A/chicken/Kurgan/05/2005 (H5N1) although the sample HR15834-C-90 showed the lowest activity. The sample HR15834-L-90 showed similar activity. An extract from intact roots showed the highest activity, IC50 of which was 93.0 µg mL−1.
The virus-inhibiting activity of aqueous extracts of all studied samples was either lower in comparison with the ethanolic extracts or even non-existent. For example, over the concentration range studied, a 50% inhibition (IC50) for aqueous extract of the sample HR15834-C-90 against influenza A/H5N1 virus was not achieved. Similarly, no virus-inhibiting activity of aqueous extracts of intact root samples against both the A/H3N2 strain and the A/H5N1 strain was achieved. Analysis of IC50 indicates that in all studied samples (with the exception of ethanolic extracts of intact roots), this parameter was larger against A/chicken/Kurgan/05/2005 (H5N1) than against A/Aichi/2/68 (H3N2). A study of the virus-inhibiting activity of aqueous extracts of fruits of N. tangutorum against A/Novosibirsk/RII-27192S/2020 (H3N2) strain of influenza virus (Kurskaya et al. 2022) showed that the values of IC50 and SI are similar to our data on virus-inhibiting activity of aqueous extracts against A/Aichi/2/68 (H3N2) strain (samples HR8196-L-90, HRA4-L-90, HR15834-L-90). At the same time the study of the activity of various fractions of the extract of root Isatis indigotica against A/H1N1 strain influenza virus mainly resulted in not too high SI (less than 1), however, the authors conclude that some fractions with SI of ca. 23 are promising candidates for the development of an antivirus drug (Yang et al. 2012).
According to the results of the current study, the highest values of the SI against the A/H3N2 strain of the samples HR15834-L-90 of the ethanolic extract of HR8196-L-90 and aqueous extract are 16.4 and 16.3, respectively. In terms of IS against both strains of influenza virus the ethanolic extract HR15834-L-90 appeared to be the most efficient. The SI values reached > 16.4 and 15.4 against A/Aichi/2/68 (H3N2) and A/chicken/Kurgan/05/2005 (H5N1), respectively.
Conclusions
The results obtained in this study demonstrated an efficient A. rhizogenes–mediated transformation protocol for N. schoberi to produce a HR culture using different Agrobacterium strains: A4, ATCC15834, R-1601, 8196 RT. Among A. rhizogenes strains studied, ATCC15834 possessed the highest potential to mass-produce genetically modified roots. It was established that the extracts of N. schoberi exhibited a low toxicity for MDCK cell culture. For the majority of the samples, CC50 was > 5.0 mg mL−1, and MTC at 1.0 mg mL−1. This study indicated that the highest antiviral activity has the ethanolic extract HR15834-L-90 and aqueous extract HR8196-L-90. Their IN were 3.50 and 6.75, respectively, whereas SI values were in the range of 12.4 to 16.4. These results call for future studies of the antiviral properties of HR extracts and the development of novel antiinfluenza drugs. The resulting HR cultures are a potential source of secondary plant metabolites with antiviral activities against the influenza virus.
Change history
24 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11627-023-10369-x
References
Ambros EV, Kotsupiy OV, Kukushkina TA, Zheleznichenko TV, Novikova TI (2020) Synthesis of biologically active compounds in hairy roots of Astragalus penduliflorus Lam. Chem Plant Raw Mater 2:209–221
Bahadori F, Javanbakht A (2006) Effect of pre-treatments on seed germination and seedling growth of Bunnium persicum of Semnan. Iran J Rang Forests Pl Breed Gen Res 14:163–169. https://doi.org/10.22092/ijrfpbgr.2006.115064
Bai J, Meng Y, Gou R, Lyu J, Dai Z, Diao X, Zhang H, Luo Y, Zhu X, Lin G (2021) Mangrove diversity enhances plant biomass production and carbon storage in Hainan island, China. Func Ecol 35:774–786. https://doi.org/10.1111/1365-2435.13753
Boubaker J, Bzeouich IM, Nasr N, Ghozlen HB, Mustapha N, Ghedira K, Chekir-Ghedira L (2015) Phytochemical capacity of Nitraria retusa leaves extracts inhibiting growth of melanoma cells and enhancing melanogenesis of B16F10 melanoma. BMC Comp Altern Med 15:300. https://doi.org/10.1186/s12906-015-0743-z
Brighente IMC, Dias M, Verdi LG, Pizzolatti MG (2007) Antioxidant activity and total phenolic content of some Brazilian species. Pharm Biol 45:156–161. https://doi.org/10.1080/13880200601113131
Cannell JJ, Zasloff M, Garland CF, Scragg R, Giovannucci E (2008) On the epidemiology of influenza. Virol J 5:422X-X425. https://doi.org/10.1186/1743-422X-5-29
Cheng D, Sun L, Zou S, Chen J, Mao H, Zhang Y, Liao N, Zhang R (2019) Antiviral effects of Houttuynia cordata polysaccharide extract on Murine Norovirus-1 (MNV-1)-A Human Norovirus surrogate. Molecules 24:1835. https://doi.org/10.3390/molecules24091835
Dunstan DI, Short KC (1977) Improved growth of tissue cultures of onion, Allium cepa. Physiol Plant 41:70–72. https://doi.org/10.1111/j.1399-3054.1977.tb01525.x
El-Esawi MA, Elkelish A, Elansary HO, Ali HM, Elshikh M, Witczak J, Ahmad M (2017) Genetic transformation and hairy root induction enhance the antioxidant potential of Lactuca serriola L. Oxid Med Cell Longev 5604746. https://doi.org/10.1155/2017/5604746
Estruch JJ, Chriqui D, Grossmann K, Schell J, Spena A (1991) The plant oncogene rolC is responsible for the release of cytokinins from glucoside conjugates. EMBO J 10:2889–2895. https://doi.org/10.1002/j.1460-2075.1991.tb07838.x
Fukazawa H, Suzuki T, Wakita T, Murakami Y (2012) A cell-based, microplate colorimetric screen identifies 7,8-benzoflavone and green tea gallate catechins as inhibitors of the hepatitis C virus. Biol Pharm Bull 35:1320–1327. https://doi.org/10.1248/bpb.b12-00251
Glezen WP (2008) Clinical practice. Prevention and treatment of seasonal influenza. N Engl J Med 359:2579–2585. https://doi.org/10.1056/NEJMcp0807498
Godoi AM, Carla Faccin-Galhardi L, Rechenchoski DZ, Arruda TBMG, Cunha AP, Almeida RR, Rodrigues FEA, Ricardo NMPS, Nozawa C, Linhares REC (2019) Structural characterization and antiviral activity of pectin isolated from Inga spp. Int J Biol Macromolec 139:925–931. https://doi.org/10.1016/j.ijbiomac.2019.07.212
Habriev RU (2005) Guideline for experimental (preclinical) studying of new pharmacological substances, 2nd edn. Medicine, Moscow In Russian
Henss L, Scholz T, von Rhein C, Wieters I, Borgans F, Eberhardt FJ, Zacharowski K, Ciesek S, Rohde G, Vehreschild M, Stephan C, Wolf T, Hofmann-Winkler H, Scheiblauer H, Schnierle S (2021) Analysis of humoral immune responses in patients with severe acute respiratory syndrome Coronavirus 2 infection. J Infect Dis 4(223):56–61. https://doi.org/10.1093/infdis/jiaa680
Hong SB, Peebles CA, Shanks JV, San KY, Gibson SI (2006) Terpenoid indole alkaloid production by Catharanthus roseus hairy roots induced by Agrobacterium tumefaciens harboring rol ABC genes. Biotechnol Bioeng 93(2):386–390. https://doi.org/10.1002/bit.20699
Hudson JB (2009) The use of herbal extracts in the control of influenza. J Med Plant Res 3:1189–1195. https://doi.org/10.5897/JMPR.9001242
Isaacs CE, Wen GY, Xu W, Jia JH, Rohan L, Corbo C, Di Maggio V, Jenkins EC Jr, Hillier S (2008) Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob Agents Chemother 52:962–970. https://doi.org/10.1128/AAC.00825-07
Kapoor R, Sharma B, Kanwar SS (2017) Antiviral phytochemicals: an overview. Biochem Physiol 6:2–8. https://doi.org/10.4172/2168-9652.1000220
Kim M, Kim SY, Lee HW, Shin JS, Kim P, Jung YS, Jeong HS, Hyun JK, Lee CK (2013) Inhibition of influenza virus internalization by (-)-epigallocatechin-3-gallate. Antiv Res 100:460–472. https://doi.org/10.1016/j.antiviral.2013.08.002
Kuluev BR, Vershinina ZR, Knyazev AV, Chemeris DA, Baimiev AK, Chumakov MI, Baimiev AK, Chemeris AV (2015) Plant “Hairy” roots are important instrumentation for researchers and powerful phytochembiofactory for manufacturers. Biomics 2:70-120. https://www.elibrary.ru/item.asp?id=24252219 In Russian
Kurskaya O, Prokopyeva E, Bi H, Sobolev I, Murashkina T, Shestopalov A, Wei L, Sharshov K (2022) Anti-influenza activity of medicinal material extracts from Qinghai-Tibet plateau. Viruses 14:360. https://doi.org/10.3390/v14020360
Kuzuhara T, Iwai Y, Takahashi H, Hatakeyama D, Echigo N (2009) Green tea catechins inhibit the endonuclease activity of influenza a virus RNA polymerase. PLoS Curr 1:1052. https://doi.org/10.1371/currents.rrn1052
Le Flem-Bonhomme V, Laurain-Mattar D, Fliniaux MA (2004) Hairy root induction of Papaver somniferum var. album, a difficult-to-transform plant by A. rhizogenes LBA 9402. Planta 218:890–893. https://doi.org/10.1007/s00425-003-1196-z
Leduc M, Tikhomiroff C, Cloutier M, Perrier M, Jolicoueyr M (2006) Development of a kinetic metabolic model: application to Catharanthus roseus hairy root. Bioprocess Biosyst Eng 28:295–313. https://doi.org/10.1007/s00449-005-0034-z
Lee SY, Kim SG, Song WS, Kim YK, Park N, Park SU (2010) Influence of different strains of Agrobacterium rhizogenes on hairy root induction and production of alizarin and purpurin in Rubia akane Nakai. Rom Biotechnol Lett 15:5405–5409. URL: https://www.rombio.eu/rbl4vol15/6/20Sook/20Young/20Lee.pdf (Accessed 1 February 2023)
Lyu SY, Rhim JY, Park WB (2005) Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch Pharm Res 28:1293–1301. https://doi.org/10.1007/BF02978215
Macias AE, McElhaney JE, Chaves SS, Nealon J, Nunes MC, Samson SI, Seet BT, Weinke T, Yu H (2021) The disease burden of influenza beyond respiratory illness. Vaccine 15:A6–A14. https://doi.org/10.1016/j.vaccine.2020.09.048
Mahy BWJ, Kangro HO (1996) Virology Methods Manual. Academic Press, London
Matkowski A, Zielinska S, Oszmianski J, Lamer-Zarawska E (2008) Antioxidant activity of extracts from leaves and roots of Salvia miltiorrhiza Bunge. S. przewalskii Maxim., and S. verticillata L. Bioresour Technol 99:7892–7896. https://doi.org/10.1016/j.biortech.2008.02.013
McComb EA, McCready RM (1952) Colorimetric determination of pectic substances. Anal Chem 24:1630–1632. https://doi.org/10.1021/ac60070a036
Mironov AN (2012) The guidelines for preclinical studies of drugs. M.: Grif and K, 944 p. https://www.elibrary.ru/item.asp?id=21532120 In Russian
Mohamed AA, Ali SI, El-Baz FK, El-Senousy WM (2015) New insights into antioxidant and antiviral activities of two wild medicinal plants: Achillea fragrantissima and Nitraria retusa. Int J Pharm Bio Sci 6:708-722. URL: https://www.ijpbs.net/abstract.php?article=Mzg5OQ (Accessed 1 February 2023)
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/J.1399-3054.1962.TB08052.X
Ninfali P, Antonelli A, Magnani M, Scarpa ES (2020) Antiviral properties of flavonoids and delivery strategies. Nutrients 12:2534. https://doi.org/10.3390/nu12092534
Novikova T, Ambros E, Kotsupiy O, Shaldaeva T, Protsenko M, Filippova E, Mazurkova N (2022) Astragalus penduliflorus Lam. hairy roots: a promising biotechnological platform for obtaining anti-influenza drugs. S Afr J Bot 147:63–70. https://doi.org/10.1016/j.sajb.2021.12.036
Novikova T, Ambros E, Zheleznichenko T, Zaitseva Y, Oyunbileg Y (2020) Agrobacterium rhizogenes-transformed roots of Astragalus membranaceus (Fisch. ex Link) Bunge as a source of valuable secondary metabolites. BIO Web of Conf 24:00064. https://doi.org/10.1051/bioconf/20202400064
Paunescu A (2011) Dormancy breakdown and germination requirments of Nitraria schoberi L. an extreme halophite plant: implication for restoration. research people and actual tasks on multidisciplinary sciences. Proceedings of the Third International Conference, Bulgaria: Lozenec, 178–182. URL: https://www.cabdirect.org/cabdirect/abstract/20143000727 (Accessed 1 February 2023)
Pavlova OA, Matveyeva TV, Lutova LA (2014) rol-Genes of Agrobacterium rhizogenes. Russ J Genet Appl Res 4:137–145. https://doi.org/10.1134/S2079059714020063
Pisarev DI, Martynova NA, Netrebenko NN, Novikov OO, Sorokopudov VN (2009) Saponins and their determination in the rhizomes of Manchurian aralia in the conditions of the Belgorod region. Khim Rast Syr 4:197-198. https://www.elibrary.ru/item.asp?id=13001133 In Russian
Rebrova OY (2006) Statistical analysis of medical data. Application of the STATISTICA software package Media Sphera, Moskow
Rothberg MB, Haessler SD, Brown RB (2008) Complications of viral influenza. Am J Med 121:258–264. https://doi.org/10.1016/j.amjmed.2007.10.040
Saha RK, Takahashi T, Kurebayashi Y, Fukushima K, Minami A, Kinbara N, Ichitani M, Sagesaka YM, Suzuki T (2010) Antiviral effect of strictinin on influenza virus replication. Antivir Res 88:10–18. https://doi.org/10.1016/j.antiviral.2010.06.008
Sathasivam R, Choi M, Radhakrishnan R, Kwon H, Yoon J, Yang SH, Kim JK, Chung YS, Park SU (2022) Effects of various Agrobacterium rhizogenes strains on hairy root induction and analyses of primary and secondary metabolites in Ocimum basilicum. Front Plant Sci 17(13):983776. https://doi.org/10.3389/fpls.2022.983776
Sawada H, Ieki H, Matsuda I (1995) PCR detection of Ti and Ri plasmids from phytopathogenic Agrobacterium strains. Appl Environ Microbiol 2:828–831. https://doi.org/10.1128/aem.61.2.828-831.1995
Sharan S, Sarin NB, Mukhopadhyay K (2019) Elicitor-mediated enhanced accumulation of ursolic acid and eugenol in hairy root cultures of Ocimum tenuiflorum L. is age, dose, and duration dependent. S Afr J Bot 124:199–210. https://doi.org/10.1016/j.sajb.2019.05.009
Sharifi-Rad J, Hoseimi-Alfatemi SM, Sharifi-Rad M, Teixeira da Silva JA (2015) Antibacterial, antioxidant, antifungal and anti-inflammatory activities of crude extract from Nitraria schoberi fruits. 3 Biotech 5:677–684. https://doi.org/10.1007/s13205-014-0266-1
Sliwi’nska A, Figat R, Zgadzaj A, Wile’nska B, Misicka A, Nał˛ecz-Jawecki G, Pietrosiuk A, Sykłowska-Baranek K (2022) Polyscias filicifolia (Araliaceae) hairy roots with antigenotoxic and anti-photogenotoxic activity. Molecules 27:186. https://doi.org/10.3390/molecules27010186
Smee DF, Morrison AC, Barnard DL, Sidwell RW (2002) Comparison of colorimetric and visual methods for determining anti-influenza (H1N1 and H3N2) viruses activities and toxicities of compounds. J Virol Meth 106:71–79. https://doi.org/10.1016/S0166-0934(02)00137-4
Spasova M, Philipov S, Nikolaeva-Glomb L, Galabov AS, Milkova T (2008) Cinnamoyl- and hydroxycinnamoyl amides of glaucine and their antioxidative and antiviral activities. Bioorg Med Chem 16:7457–7461. https://doi.org/10.1016/j.bmc.2008.06.010
Stankova I, Chuchkov K, Shishkov S, Kostova K, Mukova L, Galabov AS (2009) Synthesis, antioxidative and antiviral activity of hydroxycinnamic acid amides of thiazole containing amino acid. Amino Acids 37:383–388. https://doi.org/10.1007/s00726-008-0165-z
Steinmann J, Buer J, Pietschmann T, Steinmann E (2013) Anti-infective properties of epigallocatechin-3-gallate (egcg), a component of green tea. Br J Pharmacol 168:1059–1073. https://doi.org/10.1111/bph.12009
Tavassoli P, Safipour Afshar A (2018) Influence of different Agrobacterium rhizogenes strains on hairy root induction and analysis of phenolic and flavonoid compounds in marshmallow (Althaea officinalis L.). 3 Biotech 8:351. https://doi.org/10.1007/s13205-018-1375-z
Tepfer D (1984) Transformation of several species of higher plants by Agrobacterium rhizogenes: sexual transmission of the transformed genotype and phenotype. Cell 37:959–967. https://doi.org/10.1016/0092-8674(84)90430-6
Thiruvengadam M, Praveen N, Maria John KM, Seung-Hyun Kim YSY, Chung IM (2014) Establishment of Momordica charantia hairy root cultures for the production of phenolic compounds and determination of their biological activities. Plant Cell Tiss Org Cult 118:545–557. https://doi.org/10.1007/s11240-014-0506-4
Tian L (2015) Using hairy roots for production of valuable plant secondary metabolites. Adv Biochem Eng Biotechnol 149:275–324. https://doi.org/10.1007/10_2014_298
Tulyaganov TS, Allaberdiev FK (2003) Alkaloids from plants of the Nitraria genus. Structure of sibiridine. Chem Nat Comp 39:292–293. https://doi.org/10.1023/A:1025482821368
Van Beek TA (2002) Chemical analysis of Ginkgo biloba leaves and extracts. J Chromatogr 967:21–35. https://doi.org/10.1016/s0021-9673(02)00172-3
Vervliet G, Holsters M, Teuchy H, Van MM, Schell J (1975) Characterization of different plaque-forming and defective temperate phages in Agrobacterium. J Gen Virol 26:33–48. https://doi.org/10.1099/0022-1317-26-1-33
Vinterhalter B, Krstic·-Milosˇevic D, Janković T, Pljevljakušić D, Ninković S, Smigocki A, Vinterhalter D, Krstić-Milošević D, Janković T. et al. (2015) Gentiana dinarica Beck. hairy root cultures and evaluation of factors affecting growth and xanthone production. Plant Cell Tiss Org Cult 121:667-679https://doi.org/10.1007/s11240-015-0737-z
Weber C, Sliva K, von Rhein C, Kummerer BM, Schnierle BS (2015) The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antiviral Res 113:1–3. https://doi.org/10.1016/j.antiviral.2014.11.001
Williamson MP, McCormick TG, Nance CL, Shearer WT (2006) Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: potential for HIV-1 therapy. J Allergy Clin Immunol 118:1369–1374. https://doi.org/10.1016/j.jaci.2006.08.016
Yamaguchi K, Honda M, Ikigai H, Hara Y, Shimamura T (2002) Inhibitory effects of (-)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1). Antiviral Res 53:19–34. https://doi.org/10.1016/s0166-3542(01)00189-9
Yang Z, Wang Y, Zhong S, Zhao S, Zeng X, Mo Z, Qin S, Guan W, Li C, Zhong N (2012) In vitro inhibition of influenza virus infection by a crude extract from Isatis indigotica root resulting in the prevention of viral attachment. Mol Med Rep 5:793–799. https://doi.org/10.3892/mmr.2011.709
Zakaryan H, Arabyan EOA, Zandi K (2017) Flavonoids: promising natural compounds against viral infections. Arch Virol 162:2539–2551. https://doi.org/10.1007/s00705-017-3417-y
Zheleznichenko T, Banaev E, Asbaganov S, Voronkova M, Kukushkina T, Filippova E, Mazurkova N, Shishkina L, Novikova T (2018) Nitraria schoberi L. hairy root culture as a source of compounds with antiviral activity against influenza virus subtypes A(H5N1) and A(H3N2). 3 Biotech 8:260. https://doi.org/10.1007/s13205-018-1280-5
Zheleznichenko TV, Novikova TI, Banaev EV (2016) Effect of NaCl on the embryo rescue of Nitraria sibirica. Plant Cell Biotechnol Mol Biol 17:184-190. URL: https://www.ikprress.org/index.php/PCBMB/article/view/1401 (Accessed 1 February 2023)
Acknowledgements
The article dealt with the material of in vitro collection of the Laboratory of Biotechnology, CSBG representing USU 440534 “Collections of living plants indoors and outdoors.” Virology studies were carried out within the framework of the State assignment of the State Research Center of Virology and Biotechnology VECTOR of Rospotrebnadzor.
Funding
The work was carried out with the financial support of the budgetary project of the Central Siberian Botanical Garden (CSBG) no. AAAA17-117012610051–5 within the framework of the State Assignment and of the State assignment of State Research Centre of Virology and Biotechnology "Vector" of Rospotrebnadzor (30/21).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by the author.
Conflict of interest
The authors declare no competing interests.
Additional information
The original version of this article was revised: The Funding section was incomplete in the article as originally published and has been corrected.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheleznichenko, T., Voronkova, M., Asbaganov, S. et al. Impact of different Agrobacterium rhizogenes strains on secondary metabolites accumulation in Nitraria schoberi L. hairy roots and antiviral activity of their extracts against influenza virus of subtypes A (H5N1) and A (H3N2). In Vitro Cell.Dev.Biol.-Plant 59, 378–392 (2023). https://doi.org/10.1007/s11627-023-10343-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-023-10343-7