Abstract
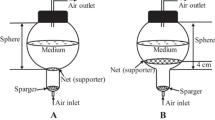
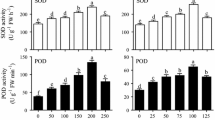
The present study designed two sets of experiments by using the uniform design method and investigated the effects of medium components on the accumulation of bioactive compounds (polysaccharide and kinsenoside) in rhizomes, in order to select a suitable culture medium for the rhizome suspension culture of Anoectochilus roxburghii (Wall.) Lindl. Among the combinations of Murashige and Skoog (MS) medium strengths and plant growth regulator (benzylaminopurine, BA; kinetin, KT; and α-naphthaleneacetic acid, NAA) concentrations, and the combinations of nitrogen, phosphorus, and sucrose concentrations, the maximum yield of polysaccharides and kinsenoside was achieved with 0.75 × MS + 2.0 mg L−1 BA + 0.2 mg L−1 KT + 0.5 mg L−1 NAA and 45 mM nitrogen + 0.93 mM phosphorus + 35 g L−1 sucrose, respectively. Therefore, the optimal rhizome suspension culture medium was 0.75 × MS medium supplemented with 2.0 mg L−1 BA, 0.2 mg L−1 KT, 0.5 mg L−1 NAA, and 35 g L−1 sucrose. Yeast extract (YE) enhanced bioactive compound accumulation in rhizomes. The polysaccharide and kinsenoside production was significantly improved when 75 mg L−1 YE was added to the culture medium after 30 d of rhizome suspension culture; 8.3 g L−1 of polysaccharide and 6.1 g L−1 of kinsenoside were obtained after 4 d of YE treatment. The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity of YE-treated rhizomes was higher than that of YE-untreated rhizomes, demonstrating enhanced antioxidant activity of the treated bioreactor-cultured rhizomes.







Similar content being viewed by others
References
Baque MA, Shiragi MHK, Moh SH, Lee EJ, Paek KY (2013) Production of biomass and bioactive compounds by adventitious root suspension cultures of Morinda citrifolia (L.) in a liquid-phase airlift balloon-type bioreactor. In Vitro Cell Dev Biol Plant 49:737–749
Cai Z, Kastell A, Smetanska I (2014) Chitosan or yeast extract enhance the accumulation of eight phenolic acids in cell suspension cultures of Malus × domestica Borkh. J Hortic Sci Biotechnol 89:93–99
Cui HY, Baque MA, Lee EJ, Paek KY (2013) Scale-up of adventitious root cultures of Echinacea angustifolia in a pilot-scale bioreactor for the production of biomass and caffeic acid derivatives. Plant Biotechnol Rep 7:297–308
Dubios M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Hajati RJ, Payamnoor V, Bezdi KG, Chashmi NA (2016) Optimization of callus induction and cell suspension culture of Betula pendula Roth for tmproved production of betulin, betulinic acid, and antioxidant activity. In Vitro Cell Dev Biol Plant 52:400–407
Han MH, Yang XW, Jin YP (2008) Novel triterpenoid acyl esters and alkaloids from Anoectochilus roxburghii. Phytochem Anal 19:438–443
He CN, Wang CL, Guo SX, Xiao PG (2004) Research progress on chemical constituents and pharmacological activities of Anoectochilus. Chin Pharm J 39:81–84
Hsiao HB, Hsieh CC, Wu JB, Lin H, Lin WC (2016) Kinsenoside inhibits the inflammatory mediator release in a type-II collagen induced arthritis mouse model by regulating the T cells responses. BMC Complem Altern M 16:80–91
Jang HR, Lee HJ, Shohael AM, Park BJ, Paek KY, Park SY (2016) Production of biomass and bioactive compounds from shoot cultures of Rosa rugosa using a bioreactor culture system. Hortic Environ Biotechnol 57:79–87
Jeong CS, Murthy HN, Hahn EJ, Lee HL, Paek KY (2009) Inoculum size and auxin concentration influence the growth of adventitious roots and accumulation of ginsenosides in suspension cultures of ginseng ( Panax ginseng C.A. Meyer). Acta Physiol Plant 31:219–222
Jiang YJ, Piao XC, Liu JS, Jiang J, Lian ZX, Kim MJ, Lian ML (2015) Bioactive compound production by adventitious root culture of Oplopanax elatus in balloon-type airlift bioreactor systems and bioactivity property. Plant Cell Tissue Organ Cult 123:413–425
Kochan E, Szymczyk P, Kuzma L, Lipert A, Szymanska G (2017) Yeast extract stimulates ginsenoside production in hairy root cultures of American ginseng cultivated in shake flasks and nutrient sprinkle bioreactors. Molecules 22:880–894
Kwiecien I, Szydlowska A, Kawka B, Beerhues L, Ekiert H (2015) Accumulation of biologically active phenolic acids in agitated shoot cultures of three Hypericum perforatum cultivars: ‘Elixir’, ‘Helos’ and ‘Topas’. Plant Cell Tissue Organ Cult 123:273–281
Lage DD, Tirado MD, Vanicore SR, Sabino KCD, Albarello N (2015) Production of betalains from callus and cell suspension cultures of Pereskia aculeata Miller, an unconventional leafy vegetable. Plant Cell Tissue Organ Cult 122:341–350
Li M, Hirata Y, Xu GJ, Niwa M (1990) Determination of polysaccharide contents in the drugs of Dendrobium. Chin Tradit Herbal Drugs 21:10–20
Li Q, Tang M, Tan YY, Ma DW, Wang YN, Zhang H (2016) Improved production of chlorogenic acid from cell suspension cultures of Lonicera macranthoids. Trop J Pharm Res 15:919–927
Lin CC, Huang PC, Lin JM (2000) Antioxidant and hepatoprotective effects of Anoectochilus formosanus and Gynostemma pentaphyllum. Am J Chin Med 28:87–96
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Rajendran L, Suvarnalatha G, Ravishankar G, Venkataraman L (1994) Enhancement of anthocyanin production in callus cultures of Daucus canota L. under the influence of fungal elicitors. Appl Microbiol Biotechnol 42:227–231
Sarikurkcu C, Tepe B, Daferera D, Polissiou M, Harmandar M (2008) Studies on the antioxidant activity of the essential oil and methanol extract of Marrubium globosum subsp. globosum (Lamiaceae) by three different chemical assays. Biores Technol 99:4239–4246
Sherif NA, Kumar TS, Rao MV (2016) In vitro regeneration by callus culture of Anoectochilus elatus Lindley, an endangered terrestrial jewel orchid. In Vitro Cell Dev Biol Plant 52:72–80
Wu CH, An D, Sun LN, Wang M, Chang GN, Zhao CY, Lian ML (2017) A novel co-culture system of adventitious roots of Echinacea species in bioreactors for high production of bioactive compounds. Plant Cell Tissue Organ Cult 130:301–311
Wungsintaweekul J, Choo-Malee J, Charoonratana T, Keawpradub N (2012) Methyl jasmonate and yeast extract stimulate mitragynine production in Mitragyna speciosa (Roxb.) Korth. shoot culture. Biotechnol Lett 34:1945–1950
Xu CP, Yun JW (2013) Optimization of submerged-culture conditions for mycelial growth and exo-biopolymer production by Auricularia polytricha (wood ears fungus) using the methods of uniform design and regression analysis. Biotechnol Appl Biochem 38:193–199
Xu YY, Zhang GF, Wang Y, Guo G (2016) Effect of La(NO3)3 and Ce(NO3)3 on shoot induction and seedling growth of in vitro cultured Anoectochilus roxburghii. J Plant Biol 59:105–113
Yang F, Lian ML, Li M, Piao XC (2016) Multiple shoot culture and effective material production of Anoectochilus roxburghii. Chin Tradit Herb Drugs 47:3284–3288
Zeng S (2005) Uniform design and its application. Medicine Technology Press, Beijing
Zeng BY, Su MH, Chen QX, Chang Q, Wang W, Li HH (2016) Antioxidant and hepatoprotective activities of polysaccharides from Anoectochilus roxburghii. Carbohyd Polym 153:391–398
Zhang Q (1995) On the comparison of orthogonal design and uniform design (I). Appl stat Manage 14:25–29
Zhang Y, Cai JH, Ruan HL, Pi HF, Wu JZ (2007) Antihyperglycemic activity of kinsenoside, a high yielding constituent from Anoectochilus roxburghii in streptozotocin diabetic rats. J Ethnopharm 114:141–145
Zhang J, Jin DC, Jin HL, Liu QG (2011a) Optimization of callus induction medium for Schisandga chinensis Baill via uniform design. Agric Sci Technol 12:141–143
Zhang JW, Tang F, Zhang XQ, Xu JT, Zhang L, Cai JY, Zhang YH (2011b) Determination of the content of kinsenoside in Anoectochilus roxburghii (Wall.) Lindl by HPLC. Chin Hosp Pharm J 31:261–263
Zhang JC, Zhang C, Chen Q, Lin CH (2014) Industrial status and development countermeasures of Anoectochilus spp. J Fujian Forestry Sci Technol 41:221–224
Zhang AL, Wang HZ, Shao QS, Xu MJ, Zhang WS, Li MY (2015) Large scale in vitro propagation of Anoectochilus roxburghii for commercial application: Pharmaceutically important and ornamental plant. Ind Crops Prod 70:158–162
Zhang W, Piao XC, Li J, Jin Y, Lian ML (2017) Optimized culture medium for the production of flavonoids from Orostachys cartilaginea V.N. Boriss. callus cultures. In Vitro Cell Dev Biol Plant 53:1–11
Funding
Project 31660080 was supported by the National Natural Science Foundation of China and project no. 23 of 2017 was supported by Youth Foundation of Yanbian University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Randall P. Niedz
Rights and permissions
About this article
Cite this article
Jin, M.Y., Zhang, L.Q., Piao, X.C. et al. Optimization of culture conditions for the production of polysaccharides and kinsenoside from the rhizome cultures of Anoectochilus roxburghii (Wall.) Lindl.. In Vitro Cell.Dev.Biol.-Plant 54, 25–35 (2018). https://doi.org/10.1007/s11627-017-9883-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-017-9883-9