Abstract
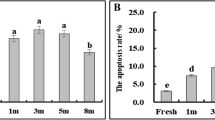
Oxidative stress induced by excessive accumulation of reactive oxidative species (ROS) during cryopreservation is thought to be one factor contributing to cryodamage of biological materials. To explore the role of oxidative stress in the cryopreservation of plant pollen, germination, ROS, and malondialdehyde (MDA) levels of pollen from 20 ornamental plant species were compared before and after cryopreservation. The results showed that the germinability of cryopreserved pollen from 13 out of the 20 species was not significantly different from that of fresh pollen (group 1), only one increased significantly, while the other six declined significantly (group 2). The MDA content in cryopreserved pollen from nine species in group 1 showed no significant difference from that of fresh pollen, while four species in group 2 rose significantly. This suggested that pollen viability and MDA levels were negatively correlated. ROS generation in cryopreserved pollen from nine species in group 1 was unchanged compared to fresh pollen, while five species in group 2 increased significantly. This suggested that pollen viability was negatively correlated with ROS generation. Additionally, both ROS and MDA levels in pollen from four species in group 2 increased significantly. In conclusion, pollen from most species possesses some cryostorage tolerance, but some species are severely damaged by cryostorage. Oxidative stress induced by the cryostorage in liquid nitrogen (LN) may be a key factor for the decreased viability in pollen following cryopreservation.
Similar content being viewed by others
References
Alba V, Bisignano V, Alba E, De Stradis A, Polignano GB (2011) Effects of cryopreservation on germinability of olive (Olea europaea L.) pollen. Genet Resour Crop Evol 58:977–982
Barnabás B, Rajki E (1976) Storage of maize (Zea mays L.) pollen at −196°C in liquid nitrogen. Euphytica 25:747–752
Benson EE, Withers LA (1987) Gas chromatographic analysis of volatile hydrocarbon production by cryopreserved plant tissue cultures: a nondestructive method for assessing stability. Cryo Letters 8:35–46
Chaudhury R, Malik SK, Rajan S (2010) An improved pollen collection and cryopreservation method for highly recalcitrant tropical fruit species of mango (Mangifera indica L.) and litchi (Litchi chinensis Sonn.) Cryo Letters 31:268–278
Chen GQ, Ren L, Zhang J, Reed BM, Zhang D, Shen XH (2015) Cryopreservation affects ROS-induced oxidative stress and antioxidant response in Arabidopsis seedlings. Cryobiology 70:38–47
Demeke T, Hughes HG (1991) Germination and storage of pollen Phytolacca dodecandra L. (endod). Ann Bot 68:13–15
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
Fang J, Wetten A, Johnston J (2008) Headspace volatile markers for sensitivity of cocoa (Theobroma cacao L.) somatic embryos to cryopreservation. Plant Cell Rep 27:453–461
Fleck RA, Benson EE, Bremner DH, Day JG (2000) Studies of free radical-mediated cryoinjury in the unicellular green alga Euglena gracilis using a non-destructive hydroxyl radical assay: a novel approach for developing protistan cryopreservation strategies. Free Rad Res 32:157–170
Ganeshan S, Rajasekharan PE (2005) Conservation and management of haploid genetic diversity through pollen cryopreservation. J Palynol 41:39–48
Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142:231–255
Jia MX, Di W, Liu Y, Shi Y, Xie YR (2016) ROS-induced oxidative stress in nobile-type Dendrobium protocorm-like bodies (PLBs) during vitrification. Cryo Letters 37:253–263
Kaczmarczyk A, Funnekotter B, Menon A, Phang PY, Al-Hanbali A, Bunn E, Mancera RL (2012) Current issues in plant cryopreservation. In: Katkov II (ed) Current frontiers in cryobiology, InTech, pp 417–438
Karipidis C, Olympios C, Passam HC, Savvas D (2007) Effect of moisture content of tomato pollen stored cryogenically on in vitro germination, fecundity and respiration during pollen tube growth. J Hortic Sci Biotechnol 82:29–34
Karun A, Sajini KK, Niral V, Amarnath CH, Remya P, Rajesh MK, Samsudeen K, Jerard BA, Engelmann F (2014) Coconut (Cocos nucifera L.) pollen cryopreservation. Cryo Letters 35:407–417
Li B, Wang H, Liu Y (2010) Pollen cryopreservation of Japanese tree peony cultivars. J Beijing For Univ 32:297–300
Li BL (2010) Studies on differentially expressed protein of pollen cryopreservation and cryobank construction of Paeonia spp. Dissertation, Beijing Forestry University
Li BL,Wang H, Liu Y (2011) Pollen cryopreservation of Camellia. Acta Hort 908:265–268
Li G (2005) Studies on cryopreservation of Camellia pollen. Dissertation, Beijing Forestry University
Li HS, Sun Q, Zhao SJ, Zhang WH (2000) Experiment principle and technology of plant physiology and biochemistry. Higher Education Press, Beijing, pp 167–261
Liu Q, Xu J, Shi Y,Meng X, Liu Y (2014) Storage methods of 12 species of lilac (Syringa L.) pollen. Acta Agriculturae Zhejiangensis 26:615–620
Liu XD, Li XD, Wu YL, Shen XH (2013) Physiological and biochemical characteristics of Dendrobium wardianum protocorms during cryopreservation. J Northeast For Univ 41:79–84
Marchant R, Power JB, Davey MR, Chartier-Hollis JM, Lynch PT (1992) Cryopreservation of pollen from two rose cultivars. Euphytica 66:235–241
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Mortazavi SMH, Arzani K, Moieni A (2010) Optimizing storage and in vitro germination of date palm (Phoenix dactylifera) pollen. J Agric Sci Technol 12:181–189
Poobathy R, Sinniah UR, Xavier R, Subramaniam S (2013) Catalase and superoxide dismutase activities and the total protein content of protocorm-like bodies of Dendrobium sonia-28 subjected to vitrification. Appl Biochem Biotechnol 170:1066–1079
Reed BM, Uchendu E, Normah M (2015) Are antioxidants effective for reducing oxidative stress during cryopreservation. In: first international symposium for In Vitro conservation and cryopreservation, at Tepatitlan de Morolos, Jalisco, Mexico (Vol. 1)
Ren L, Zhang D, Jiang X, Gai Y, Wang W, Reed BM (2013) Peroxidation due to cryoprotectant treatment is a vital factor for cell survival in Arabidopsis cryopreservation. Plant Sci 212:37–47
Shang XQ (2005) Studies on cryopreservation of peony pollen. Dissertation, Beijing Forestry University
Shang XQ, Tao QB, Liu Y (2004) Studies on the changes of some physiological properties of tree peony pollen after cryopreservation. In: Zhang Q (ed) Advances in ornamental horticulture of China. China Forestry Publishing House, Shanghai, pp 197–199
Shi Y, Jia MX, Di W, Liu Y (2015) Research on the change of reactive oxygen species of peony pollen during cryopreservation. J Northwest For Univ 30:86–90
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295
Subbarayan K, Rolletschek H, Senula A, Ulagappan K, Hajirezaei MR, Keller ERJ (2015) Influence of oxygen deficiency and the role of specific amino acids in cryopreservation of garlic shoot tips. BMC Biotechnol 15:40
Tao QB (2003) Studies on cryopreservation of tree peony pollen. Dissertation, Beijing Forestry University
Tyagi RK, Hymowitz T (2003) Pollen from Glycine species survive cryogenic exposure. Cryo Letters 24:119–124
Uchendu EE, Leonard SW, Traber MG, Reed BM (2010) Vitamins C and E improve regrowth and reduce lipid peroxidation of blackberry shoot tips following cryopreservation. Plant Cell Rep 29:25–35
Varghese B, Naithani SC (2008) Oxidative metabolism-related changes in cryogenically stored neem (Azadirachta indica A. Juss) seeds. J Plant Physiol 165:755–765
Vendrame WA, Carvalho VS, Dias JMM, Maguire I (2008) Pollination of Dendrobium hybrids using cryopreserved pollen. Hortscience 43:264–267
Wang JF, Liu YX, Liu XJ, Lin SQ (2004) Dry freezing cryopreservation of loquat pollen. Chin Agric Sci Bull 20(1–2):20
Wen B, Wang RL, Cheng HY, Song SQ (2010) Cytological and physiological changes in orthodox maize embryos during cryopreservation. Protoplasma 239:57–67
Whitaker C, Beckett RP, Minibayeva FV, Kranner I (2010) Production of reactive oxygen species in excised, desiccated and cryopreserved explants of Trichilia dregeana Sond. S Afr J Bot 76:112–118
Xu J (2014) A study on the mechanism of Magnolia denudata pollen cryopreservation. Dissertation, Beijing Forestry University
Xu J, Li BL, Liu Q, Shi Y, Peng JG, Jia MX, Liu Y (2014a) Wide-scale pollen banking of ornamental plants through cryopreservation. Cryo Letters 35:312–319
Xu J, Liu Q, Jia MX, Liu Y, Li BL, Shi Y (2014b) Generation of reactive oxygen species during cryopreservation may improve Lilium×siberia pollen viability. In Vitro Cell Dev Biol Plant 50:369–375
Zhang YL (2007) Pollen cryopreservation of Prunus mume Sieb. et. Zucc. and the construction of pollen bank. Dissertation, Beijing Forestry University
Zhang YL, Shang XQ, Liu Y (2006) Advances in research of pollen cryopreservation. J Beijing For Univ 28:139–147
Zhang YL, Chen RD, Huang CJ, Liu Y (2009) Cryo-banking of Prunus mume pollen and its application in cross-breeding. Cryo Letters 30:165–170
Zhao Y, Qi LW, Wang WM, Saxena PK, Liu CZ (2011) Melatonin improves the survival of cryopreserved callus of Rhodiola crenulata. J Pineal Res 50:83–88
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 31370693).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Barbara Reed
Rights and permissions
About this article
Cite this article
Jia, M.X., Shi, Y., Di, W. et al. ROS-induced oxidative stress is closely related to pollen deterioration following cryopreservation. In Vitro Cell.Dev.Biol.-Plant 53, 433–439 (2017). https://doi.org/10.1007/s11627-017-9844-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-017-9844-3