Abstract
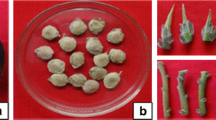
Brachystelma glabrum Hook.f. is an endemic plant species of Eastern Ghats, India. In this study, efficient protocols for in vitro micropropagation, flowering, and tuberization of this plant were developed. Sterilized shoot tip and nodal explants were cultured on Murashige and Skoog (MS) medium supplemented with different plant growth regulators (PGRs) and additives for shoot induction and multiplication. Both shoot tip and nodal explants showed the best response (90 and 100%, respectively) on MS medium supplemented with thidiazuron (TDZ) at 1.0 mg L−1. The microshoots multiplied best on MS + TDZ (1.0 mg L−1) in combination with α-naphthaleneacetic acid (NAA) at 0.5 mg L−1 and coconut water (CW) at 25%. The highest number of in vitro flowers (4.0 flowers per microshoot) was observed on MS medium supplemented with a combination of N6-benzyladenine (BA) and indole-3-butyric acid (IBA), each at 1.5 mg L−1. In vitro-derived shoots produced aerial tubers on MS + TDZ (2.0 mg L−1) + IBA (0.5 mg L−1) and basal tubers on MS + TDZ at 2.0 mg L−1. In vitro shoots were best rooted on half-strength (½) MS + NAA at 0.5 mg L−1. The rooted plantlets were successfully acclimatized in pots with 70% survival after a hardening period of 1 mo. This protocol provides an effective method for the conservation of this endemic plant species.


Similar content being viewed by others
References
Abdulmalik MM, Usman IS, Olarewaju JD, Aba DA (2012) Effect of naphthalene acetic acid (NAA) on in vitro rooting of regenerated microshoots of groundnut (Arachis hypogaea L.). Bayero J Pure Appl Sci 5(2):128–131
Al Malki AAHS, Suliman Elmeer KM (2009) Effect of medium strength and charcoal combined with IBA and NAA on root initiation of Ficus anastasia. Acad J Plant Sci 2:169–172
Atique Akbar M, Karmakar BK, Roy SK (2003) Callus induction and high-frequency plant regeneration of pineapple (Anonas comosus (L.) Merr.). Plant Tiss Cult 13(2):109–116
Avenido RA, Haulea DM (1990) In vitro organogenesis and flowering in mung bean (Vigna radiata L.). Philipp J Crop Sci 15:169–173
Bagadekar AN, Jayaraj M (2011) In vitro flowering of Heliotropium indicum L.—an important medicinal herb. Asian J Exp Biol Sci 2:90–95
Bruyns PV (2009) Three new species of Brachystelma (Apocynaceae, Asclepiadoideae, Ceropegieae) from South Tropical and Southern Africa. Novon 19:18–22
Erdaǧ BB, Emek YÇ (2009) Adventitious shoot regeneration and in vitro flowering of Anthemis xylopoda O.Schwarz, a critically endangered Turkish endemic. Turk J Biol 33:319–326
Faisal M, Anis M (2005) Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult 80:187–190
Fatima N, Anis M (2012) Role of growth regulators on in vitro regeneration and histological analysis in Indian ginseng (Withania somnifera L.) Dunal. Physiol Mol Biol Plants 18:59–67
Franklin G, Pius PK, Ignacimuthu S (2000) Factors affecting in vitro flowering and fruiting of green pea (Pisum sativum L.). Euphytica 115:65–73
George EF (1993) Plant propagation by tissue culture, vol 1. Exegetics Ltd, Basingstoke
Hussain MK, Anis M (2006) Rapid in vitro propagation of Eclipta alba (L.) Hassk. through high frequency axillary shoot proliferation. Acta Physiol Plant 28:25–330
Hussain TM, Chandrasekhar T, Gopal GR (2008) Micropropagation of Sterculia urens Roxb., an endangered tree species from intact seedlings. Afr J Biotechnol 7(2):95–101
Hussey G, Stacey NJ (1984) Factors effecting the formation of in vitro tubers of potato (Solanum tuberosum L.). Ann Bot 53:565–578
Ignacimuthu S, Franklin G, Melchias G (1997) Multiple shoot formation and in vitro fruiting of Vigna mungo L. Hepper. Curr Sci 73:733–735
Islam MT, Keller ERJ, Philibert D (2008) Effect of growth regulators on in vitro propagation and tuberization of four Dioscorea species. Plant Tissue Cult Biotechnol 18:25–35
Kalimuthu K, Prabakaran R (2013) In vitro flowering from nodal explants of Ceropegia pusilla Wight and Arn. Int J Bot Res 3(3):35–42
Le CL (1999) In vitro microtuberization: an evaluation of culture conditions for the production of virus free seed potatoes. Potato Res 42:489–498
Loc NH, Duc DT, Kwon TH, Yang MS (2005) Micropropagation of zedoary (Curcuma zedoaria roscoe)—a valuable medicinal plant. Plant Cell Tissue Organ Cult 81:119–122
Mabberley DJ (2008) Mabberley’s plant-book: a portable dictionary of plants, their classification and uses. Cambridge University Press, Cambridge
Madec P (1963) Tuber forming substances in potato. In: Lins JI, Milthorpe FL (eds) Growth of the potato. Butterworth, London, pp 121–131
Mbanaso ENA, Chukwu LI, Opara MUA (2007) In vitro basal and nodal microtuberization in yam shoot cultures (Discorea rotundata poir, cv. Obiaoturugo) under nutritional stress conditions. Afr J Biotechnol 6:2444–2446
Meve U (2002) Brachystelma. In: Albers F, Meve U (eds) Illustrated handbook of succulent plants: Asclepiadaceae. Springer-Verlag, Berlin, pp 20–46
Mithila J, Hall JC, Victor JMR, Saxena K (2003) Thidiazuron induces shoot organogenesis at low concentrations and somatic embryogenesis at high concentrations on leaf and petiole explants of African violet (Saintpaulia ionantha Wendl.). Plant Cell Rep 21:408–414
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murch SJ, Saxena PK (2001) Molecular fate of thidiazuron and its effects on auxin transport in hypocotyls tissues of Pelargonium × hortorum Bailey. Plant Growth Regul 35:269–275
Murthy KSR, Kondamudi R, Karuppasamy S (2012) Microtuberization of Ceropegia spiralis Wight and Ceropegia pusilla Wt. and Arn. African J Plant Sci 6:321–327
Nasib A, Ali K, Khan S (2008) In vitro propagation of croton (Codiaeum variegatum). Pak J Bot 40:99–104
Panigrahi J, Behera M, Maharana S, Mishra RR (2007) Biomolecular changes during in vitro organogenesis of Asteracantha longifolia (L.) Nees: a medicinal herb. Indian J Biotechnol 45:911–919
Revathi Lakshmi S, Franklin Benjamin JH, Senthil Kumar T, Murthy GVS, Rao MV (2010) In vitro propagation of Hoya wightii ssp. palniensis K.T. Mathew, a highly vulnerable and endemic species of Western Ghats of Tamil Nadu. Afr J Biotechnol 9:620–627
Seema Rajaram M, Rathod J, Dilip V (2014) Pharmacognostical studies on the tuber of Brachystelma edulis Coll. and Helmsl.—an endemic to peninsular, India. World J Pharm Pharmaceut Sci 3(6):1958–1965
Shah SN, Husaini AM, Ansari SA (2013) Micropropagation of Litsea glutinosa (Lour) C.B. Global J Cell Mol Biol 1(1):46–53
Sharma R, Shahzad A (2008) Thidiazuron (TDZ) induced regeneration from cotyledonary node explant of Abelmoschus moschatus Medik. L., (a valuable medicinal plant). World J Agric Sci 4:449–452
Sheeba E, Palanivel R, Parvathi S (2015) In vitro flowering and rapid propagation of Physalis minima Linn, a medicinal plant. Int J Innov Res Sci Eng Technol 4:18763–18768
Shriram V, Nanekar V, Kumar V, Kavikishor PB (2014) In vitro regeneration and ploidy level analysis of Eulophia ochreata Lindl. Ind Exp Biol 52:1112–1121
Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissue cultured in vitro. Symp Soc Exp Biol 11:118–130
Sudhakar Reddy C, Reddy KN, Prasad PRC, Raju VS (2003) Threatened endemic plants from Eastern Ghats India. ENVIS Newsletter 9(2):3–7
Uranbey S, Ipek A, Caliskan M, Dundar E, Cocu S, Basalma D, Guneylioglu H (2010) In vitro bulblet induction from bulb scales of endangered ornamental plant Muscari azureum. Biotechnol Biotechnol Equip 24:1843–1848
Vadawale AV, Barve DM, Dave AM (2006) In vitro flowering and rapid propagation of Vitex negundo L.—a medicinal plant. Indian J Biotechnol 5:112–116
Vanderhoef L, Key JL (1968) Inhibition by kinetin of cell elongation and RNA synthesis in excised soybean hypocotyls. Plant Cell Physiol 9:343–351
Venu P, Prasad K (2015) The existential crisis in Indian Brachystelma (Apocynaceae). Scientific correspondence. Curr Sci 109:680–682
Verma R, Singh RR (2007) Regeneration and in vitro flowering in Brassica campestris L. var. Bhavani. Our Nature 5:21–24
Zhang T (2007) In vitro flowering of Perilla frutescens. In Vitro Cell Dev Biol Plant 43:91–94
Acknowledgements
The authors are thankful to Dr. S. Kaliamoorthy, the Botanical Survey of India, Southern Regional Centre, National Orchidarium, and Experimental Garden, Yercaud, Tamil Nadu, India, for help rendered during the field work; and Dr. G.V.S. Murthy, Head of the Office, Botanical Survey of India, Southern Regional Centre, Coimbatore, Tamil Nadu, India, for authentication of the plant species. The author Dr. M.V. Rao acknowledge the UGC for providing Emeritus Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Jude Grosser
Rights and permissions
About this article
Cite this article
Revathi Lakshmi, S., Parthibhan, S., Ahamed Sherif, N. et al. Micropropagation, in vitro flowering, and tuberization in Brachystelma glabrum Hook.f., an endemic species. In Vitro Cell.Dev.Biol.-Plant 53, 64–72 (2017). https://doi.org/10.1007/s11627-017-9803-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-017-9803-z