Abstract
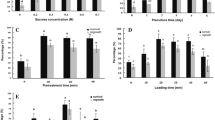
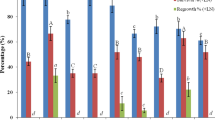
In this study, we applied the vitrification (V) cryo-plate protocol for cryopreservation of Clinopodium odorum in vitro-derived shoot tips. We studied the recovery of shoot tips after modification of various parameters of the V cryo-plate protocol, including sucrose concentration in the preconditioning medium, composition of the loading solution (LS) and vitrification solution (VS) and duration of treatment with PVS2 VS, which contained (w/v) 30% glycerol, 15% dimethylsulphoxide, 15% ethylene glycol and 13.7% sucrose. We also compared the efficiency of the V cryo-plate protocol with the dehydration (D) cryo-plate protocol. The optimal conditions determined for the V cryo-plate protocol included a 24-h preconditioning treatment on medium with 0.3 M sucrose; treatment for 20 min at room temperature with LS containing 2.0 M glycerol and 0.4 M sucrose; and dehydration with PVS2 for 60 min at 0°C. Under these conditions, 71.0% recovery of cryopreserved shoot tips was achieved. Only 29.2% regeneration was noted with the D cryo-plate protocol.







Similar content being viewed by others
References
Barboza GE, Cantero JJ, Nuñez PA, Ariza Espinar L (2009) Medicinal plants: review and a phytochemical and ethnopharmacological screening of the native Argentine flora, 34th edn. Univ Kurtziana, Nacional de Cordoba, Argentina
Barraco G, Sylvestre I, Engelmann F (2011a) Cryopreservation of sugarcane (Saccharum spp.) shoot tips using encapsulation and droplet-vitrification. Sci Hortic 130:320–324
Barraco G, Sylvestre I, Iapichino G, Engelmann F (2011b) Cryopreservation of Limonium serotinum apical shoots from in vitro plantlets using droplet-vitrification. Sci Hortic 130:309–313
Bouman H, Tiekstra A, Petutschnig E, Homan M, Schreurs R (2003) Cryopreservation of Lilium species and cultivars. Acta Hortic 612:147–154
Cantero JJ, Bianco CA (1986) Las Plantas Vasculares del Suroeste de la Provincia de Cordoba. Catalogo Preliminar de las Especies. Rev Univ Nacional De Rıo Cuarto 6:65–75 (in Spanish)
Diaz MS, Palacio L, Figueroa AC, Goleniowski ME (2012) In vitro propagation of Muña-Muña (Clinopodium odorum (Griseb.) Harley). Biotech Res Intl. doi:10.1155/2012/196583
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell Dev Biol – Plant 47:5–16
Engelmann F (2014) Cryopreservation of clonal crops: a review of key parameters. Acta Hortic 1039:31–39
Goleniowski ME, Bongiovanni GA, Palacio L, Nuñez CO, Cantero JJ (2006) Medicinal plants from the “Sierra de Comechingones”, Argentina. J Ethnopharmacol 107:324–341
Harley RM, Paucar AG (2000) List of species of tropical American Clinopodium (Labiatae), with new combinations. Kew Bull 55:917–927
Kim HH, Lee YG, Ko HC, Park SU, Gwag JG, Cho EG, Engelmann F (2009a) Development of alternative loading solutions in droplet-vitrification procedures. CryoLetters 30:291–299
Kim HH, Lee YG, Shin DJ, Kim T, Cho EG, Engelmann F (2009b) Development of alternative plant vitrification solutions in droplet-vitrification procedures. CryoLetters 30:320–334
Mahady GB (2005) Medicinal plants for the prevention and treatment of bacterial infections. Curr Pharma Design 11:2405–2427
Mari S, Engelmann F, Chabrillange N, Huet C, Michaux-Ferrière N (1995) Histo-cytological study of coffee (Coffea racemosa and C. sessiliflora) apices of in vitro plantlets during their cryopreservation using the encapsulation-dehydration technique. CryoLetters 16:289–298
Martinez GJ, Planchuelo AM, Fuentes E, Ojeda M (2006) A numeric index to establish conservation priorities for medicinal plants in the Paravachasca Valley, Cordoba, Argentina. Biodiv Conserv 15:2457–2475
Matsumoto T, Sakai A, Yamada K (1994) Cryopreservation of in vitro grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep 13:442–446
Matsumoto T, Yamamoto S, Fukui K, Niino T (2013) Cryopreservation of persimmon shoot tips using D cryo-plate procedure. In Abstract: 2nd Intl. Symp. on Plant Cryopreservation. Fort Collins, USA, 11–14 August 2013, p 96
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Niino T, Yamamoto S, Fukui K, Castillo Martinez CR, Valle Arizaga M, Matsumoto T, Engelmann F (2013) Dehydration improves cryopreservation of mat rush (Juncus decipiens Nakai) basal stem buds on cryo-plates. CryoLetters 34:549–560
Niino T, Wunna WK, Nohara N, Rafique T, Yamamoto S, Fukui K, Valle Arizaga M, Castillo Martinez CR, Matsumoto T, Engelmann F (2014) Cryopreservation of mat rush lateral buds by air dehydration using aluminum cryo-plate. Plant Biotech Rep 31:281–287
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73
Panis B, Piette B, Swennen R (2005) Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Sci 168:45–55
Panis B, Piette B, André E, Van den Houwe I, Swennen R (2011) Droplet vitrification: the first generic cryopreservation protocol for organized plant tissues? Acta Hortic 908:157–163
R Foundation for Statistical Computing (2013) R: a Language and Environment for Statistical Computing. Version 3.0.1 (2013-05-16). http://www.r-project.org. Accessed 24 Oct 2014
Sakai A, Engelmann F (2007) Vitrification, encapsulation–vitrification and droplet–vitrification: a review. CryoLetters 28:151–172
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Salma M, Fki L, Engelmann-Sylvestre I, Niino T, Engelmann F (2014) Comparison of droplet-vitrification and D-cryoplate for cryopreservation of date palm (Phoenix dactylifera L.) polyembryonic masses. Sci Hortic 179:91–97
Sekizawa K, Yamamoto S, Rafique T, Fukui K, Niino T (2011) Cryopreservation of in vitro-grown shoot tips of carnation (Dianthus caryophyllus L.) by vitrification method using aluminium cryo-plates. Plant Biotech 28:401–405
Vujovic T, Sylvestre I, Ruzic D, Engelmann F (2011) Droplet-vitrification of apical shoot tips of Rubus fruticosus L. and Prunus cerasifera Ehrh. Sci Hortic 130:222–228
Yamamoto S, Rafique T, Priyantha WS, Fukui K, Matsumoto T, Niino T (2011) Development of a cryopreservation procedure using aluminium cryo-plates. CryoLetters 32:256–265
Yamamoto S, Fukui K, Rafique T, Khan NI, Castillo Martinez CR, Sekizawa K, Matsumoto T, Niino T (2012a) Cryopreservation of in vitro-grown shoot tips of strawberry by the vitrification method using aluminium cryo-plates. Plant Genet Res: Charact Util 10:14–19
Yamamoto S, Rafique T, Fukui K, Sekizawa K, Koyama A, Ichihashi T, Niino T (2012b) Development of an effective cryopreservation protocol using aluminium cryo-plates for in vitro-grown shoot tips of mulberries (Morus spp.) originated from the tropics and subtropics. Sanshi-Konchu Biotechnol 81:57–62 (in Japanese)
Yamamoto S, Rafique T, Fukui K, Sekizawa K, Niino T (2012c) V-Cryo-plate procedure as an effective protocol for cryobanks. Case study of mint cryopreservation. CryoLetters 33:12–23
Acknowledgements
This work has been performed in the framework of the AMCRYO project, funded by Agropolis Fondation (project no. 1202–007). Dr. Maria Ester Goleniowski (CEPROCOR, Cordoba, Argentina) introduced the plant material in vitro and performed the initial multiplication of cultures. We thank Lionel Feuillassier for his assistance in performing the statistical analysis of results.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: John Forster
Rights and permissions
About this article
Cite this article
Engelmann-Sylvestre, I., Engelmann, F. Cryopreservation of in vitro-grown shoot tips of Clinopodium odorum using aluminium cryo-plates. In Vitro Cell.Dev.Biol.-Plant 51, 185–191 (2015). https://doi.org/10.1007/s11627-015-9668-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9668-y