Abstract
Tendinopathy is a common tendon disorder characterized by pain, swelling, and dysfunction. Current evidence has demonstrated that the depletion of stem cell pool and non-tenogenic differentiation of tendon-derived stem/progenitor cells (TSPCs) might account for the pathogenesis of tendinopathy. FNDC5/Irisin, as a novel exercise-induced myokine, is proved to be involved in the exercise-induced protective effects on musculoskeletal disorders. However, whether irisin can affect TSPCs fate is still unknown. To ascertain the roles of irisin on the proliferation and tenogenic differentiation of TSPCs, rat TSPCs were isolated and incubated with irisin. Cell viability, phenotypic changes, and related signaling pathways were evaluated by CCK-8 assay, colony formation assay, real-time PCR, Western blot, immunofluorescence, and proteasome activity assay. We found that irisin treatment increased the proliferative and colony-forming abilities, and promoted the tenogenic differentiation of TSPCs by upregulating the expression of YAP/TAZ. In conclusion, our work showed for the first time that irisin promotes the proliferation and tenogenic differentiation of rat TSPCs in vitro by activating YAP/TAZ, and the process was associated with a ubiquitin-proteasome proteolytic pathway. In conclusion, irisin and agents targeting YAP/TAZ may be promising therapeutic options for tendinopathy.
Similar content being viewed by others
Introduction
Tendons are tough, high-tensile-strength bands of the collagenous musculoskeletal tissues that connect muscle to bone to allow for body movement. Tendinopathy is a clinical condition characterized by pain, swelling, and dysfunction, mainly arising from overuse. Among all low limb tendinopathies, Achilles tendinopathy is the most prevalent in the general population and has a prevalence of 6.2–9.5% and 11.83% in the athletic and non-athletic population respectively (Murphy et al. 2018), causing a severe economic and social burden. Current tendinopathy management tends to support exercise-based therapy as the most evidence-based intervention (Malliaras 2017; Rio et al. 2017; Cardoso et al. 2019). The adjuncts, such as electrophysical agents, nonsteroidal anti-inflammatory drugs, corticosteroid injections, and surgery, may be required as adjuvant therapies. However, the healing process of injured tendon is usually slow and fails to regain the complete function of the normal tendon with these treatments (Gaspar et al. 2015; Liu et al. 2017).
Tendon-resident stem cells, termed tendon-derived stem/progenitor cells (TSPCs), were firstly identified in human and mouse tendons by Bi et al. in 2007 (Bi et al. 2007). At present, TSPCs have also been isolated and cultured from various species, such as rabbit, rat, and equine (Rui et al. 2010; Yin et al. 2010; Zhang and Wang 2010; Durgam et al. 2016). Similar to other classical mesenchymal stem cells (MSCs), TSPCs share some common characteristics, including surface markers, self-colony ability, and multi-lineage differentiation potential (Zhang et al. 2016). Besides, TSPCs also express tendon-related markers, such as scleraxis and tendomodulin, and can differentiate into tendon-like tissues. Therefore, the viability and tenogenic differentiation of TSPCs may play a critical role in tendon maintenance and regeneration.
Irisin is a well-defined myokine derived from proteolytic cleavage of the fibronectin type III domain-containing 5 (FNDC5) gene and is secreted mainly by skeletal muscle (Boström et al. 2012). During exercise, peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) signaling transduction activates FNDC5 expression to increase the secretion of irisin (Boström et al. 2012). Initial studies have shown that irisin can promote muscle development and browning of white adipose tissue (Boström et al. 2012). Further studies have shown that irisin plays a vital role in a variety of physiological and pathological processes such as inflammation, proliferation, aging, metastasis, and neurogenesis (Panati et al. 2016; Rabiee et al. 2020). It has been mentioned above that exercise has been regarded as the most evidence-based intervention for tendinopathy (Malliaras 2017; Rio et al. 2017; Cardoso et al. 2019). Interestingly, recent study performed by Zhang et al. showed that exercise exerted its anabolic effects on tendons by increasing TSPC proliferation and collagen synthesis in mouse (Zhang et al. 2010). This study further confirmed the beneficial effects of exercise on TSPCs in injured tendons. However, irisin as the secretory product of skeletal muscle during exercise, whether irisin has a beneficial effect on tendon repair is still unknown.
In the present study, we investigated the relationship between irisin and the proliferation and tenogenic differentiation of TSPCs. We concentrated on the phenotypic changes of TSPCs and the underlying mechanism. We hope this work will provide a novel strategy for tendon repair.
Materials and methods
Reagents
Recombinant rat irisin was purchased from R&D Systems, Abingdon, UK. Verteporfin was purchased from Selleck, Shanghai, China. Dulbecco’s modified Eagle’s medium (DMEM), penicillin/ streptomycin, fetal bovine serum (FBS), and 0.25% trypsin were obtained from Gibco BRL, Grand Island, NY. Collagenase I and ascorbic acid were purchased from Sigma-Aldrich, St, Louis, MO.
Cell culture
Achilles tendons were obtained from 3-wk-old Sprague-Dawley (SD) rats. Under sterile conditions, the tendons were cut into 1-mm3 particles, and the tissues were incubated with 0.1% type I collagenase at 37°C for 2h to isolate tendon cells. Single-cell tendon-derived cells were cultured in 48-well plates for 7 d, and colonies were collected as passage 0 (P0). The cells were passaged at a ratio of 1:3, and P3 cells were used. DMEM supplemented with 10% FBS and 100 units/ml penicillin and 100 μg/ml streptomycin was used to expand single-cell colonies. Cells were cultured at 37°C with 5% CO2. All experiments were performed with mycoplasma-free cells.
Identification of surface markers
Cells were stained with fluorescent primary antibody on ice for 40 min, washed 3 times, and detected by flow cytometry. The negative control had no fluorescent antibody. Fluorescent primary antibody: FITC anti-rat CD29, FITC anti-rat CD44, PE anti-rat CD45, PE anti-rat CD90 (Bioleague, Poggensee, Germany).
Cell viability assay
To analyze the toxicity of irisin on TSPCs, CCK-8 assay (Dojindo Molecular, Rockville, MD) was used in accordance with the manufacturer’s instruction. The cells were seeded into 96-well plates (5 × 103/well), then treated with various concentrations of irisin for 3 d. The cells were then incubated with fresh media (containing 10% CCK-8 solution) for 2h at 37°C and read at a wavelength of 450nm with a microplate spectrophotometer.
Colony formation assay
To analyze the effect of irisin on TSPC proliferation, the cells were seeded into 96-well plates (5 × 103/well), then treated with various concentrations of irisin for 3 d. The cell colony number and size were counted and calculated under a Leica optical microscope.
Western blot
After treatment, total cellular proteins were extracted with RIPA according to the manufacturer’s protocol. Nuclear Extraction Reagents (Bosterbio, Wu Han, China) were used to prepare nuclear extracts. Equal amounts of extracted proteins were separated via 10% sodium dodecyl sulfate (SDS)–polyacrylamide gels and transferred into nitrocellulose membranes. After being blocked with 5% BSA for 1h, the membranes were incubated with primary antibodies overnight at 4°C. Afterwards, the membranes were incubated with secondary antibodies at room temperature for 1 h and then detected with the Bio-Rad ChemiDoc System. All assays were performed in triplicate. The relative amount of proteins was analyzed with Quantity One software (Bio-Rad, Hercules, CA). GAPDH and TBP worked as endogenous control for total cellular and nuclear protein analysis respectively.
Real-time PCR
After treatment, total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Total RNA was used to synthesize cDNA by reverse transcription (cDNA synthesis kit, Takara, Kusatsu, Japan). Then, the cDNA samples were replicated using SYBR Premix Ex Taq II (Takara) by ABI StepOnePlus System. Each 10μl sample contained 5μl of SYBR Premix Ex Taq II, 0.4μl of each forward and reverse primer, 1μl of cDNA (10ng), and 3.2μl of ddH2O. The reaction conditions were as follows: denaturation, 95°C×30 s, followed by 40 cycles of 95°C×15 s→60°C×32 s→72°C×1 min→72°C×5 min. GAPDH was used as the endogenous control. Data were analyzed for fold difference Using the 2−ΔΔCT method. All assays were performed in triplicate. The primer pairs shown in Table 1 were used for the real-time PCR (RT-PCR) amplification.
Immunofluorescence
TSPCs cultured on 24-well plates were incubated with irisin for various times (0, 1d, 3d, 7d). After fixation with methanol, cells were permeabilized by PBS containing 0.5% v/v Triton X-100 for 20min and blocked with 5% BSA at room temperature for 2h. Cells were incubated with primary antibody against YAP at 4°C overnight, followed by being incubated with fluorescein isothiocyanate-conjugated secondary antibodies for 1h. Cell nucleuses were stained with DAPI for 5min. Then, cells were analyzed with a Leica fluorescence microscope.
Enzyme-linked immunosorbent assay (ELISA)
The endogenous irisin secretion in cells was quantified in culture media by a commercial ELISA kit (Phoenix Pharmaceuticals, Inc.; EK-067-29) according to the manufacturer. The TSPCs were cultured in the tenogenic differentiation induction medium comprising high-glucose DMEM, 10% FBS, 37.5 μg/ml ascorbic acid, and 1% penicillin G for 7 d. The medium was changed every day. The irisin levels were detected by collecting the cell culture supernates from each sample at day 1, 3, and 7. Then, absorbance from each sample was measured at the wavelength of 450nm using a spectrophotometer, and the concentrations of FNDC5/Irisin were determined by comparing the optical density (OD) values of the tested samples to that of the standard curve. All experiments were performed three times.
Proteasome activity assay
A Proteasome-GloTM Chymotrypsin-like Cell-Based Assays kit (Promega, Madison, WI) was used according to the manufacturer’s instructions. Briefly, TSPCs were seeded at 10,000 cells per well with 100μl in 96-well plates. After irisin treatment, an equal volume of luminogenic substrate specified for chymotrypsin-like protease activity was added to samples. After shaking for 2min and incubation at room temperature for 10min, luminescence was detected by a luminometer (BioTek Instruments, Winooski, VT). To confirm assay specificity, the same number of samples was pretreated for 1h with 10μM epoxomicin, a proteasome inhibitor. For each sample, proteasome activity was normalized with epoxomicin-pretreated luminescence as the background signal.
Statistical analysis
All data are presented as means ± SDs. One-way ANOVA with a subsequent post hoc Tukey’s test was used for multiple comparisons. P<0.05 is considered statistically significant.
Results
Effects of irisin on the proliferative and colony-forming abilities of rat TSPCs
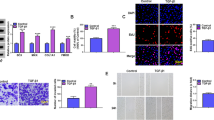
The cell surface markers analysis was performed to identify the stem status of the clonogenic cells. Results demonstrated that the clonogenic cells expressed the stem cell markers CD29, CD44, and CD99, but were negative for the leukocyte marker CD45 (Fig. 1a). To clarify if tenogenic differentiation could affect FNDC5/irisin expression and secretion in TSPCs, TSPCs were cultured in the tenogenic differentiation induction medium comprising high-glucose DMEM, 10% FBS, 37.5 μg/ml ascorbic acid, and 1% penicillin G for 7 d. The results of Western blot showed that the FNDC5 protein expression levels of TSPCs increased with the induction time (Fig. 1b–c). What’s more, we found that the secretion of irisin in TSPCs was obviously enhanced at day 1, and then it presented a decreasing tendency over the following 6-d period of the induced tenogenic differentiation (Figure S1). The cell viability assay and the colony formation assay were performed to evaluate the proliferative and colony-forming abilities of TSPCs treated with irisin of different concentrations (0, 2, 5, and 10ng/ml) for 3 d. The cell viability assay results showed that the relative cell viability of TSPCs increased with increasing concentrations of irisin (Fig. 1d). When compared to the blank group, irisin concentration of 10ng/ml could significantly increase the cell viability of TSPCs (P<0.05). The results indicated that irisin could increase the proliferative ability of TSPCs. Furthermore, the results of colony formation assay revealed that irisin did also play a positive role on the colony-forming ability of TSPCs. As shown in Fig. 1e–f, the relative colony number and size enlarged with irisin treatment in a dose-dependent manner, and significant difference was also observed at a concentration of 10ng/ml compared to the blank group. Taken together, these data confirmed the positive effects of irisin on the proliferative and colony-forming abilities of rat TSPCs.
Effects of irisin on the proliferative and colony-forming abilities of rat TSPCs. (a) The cell surface markers analysis was performed to identify the stem status of cultured TSPCs using CD29, CD 44, CD45, and CD90 (red: control; blue: fluorescent antibody). (b–c) The FNDC5 protein expression levels of TSPCs in tenogenic differentiation induction medium. (d) Cell viability of TSPCs that treated with various concentrations of irisin (0, 2, 5, and 10ng/ml) for 3 d. (e) Relative colony number of TSPCs that treated with various concentrations of irisin (0, 2, 5, and 10ng/ml) for 3 d. (f) Relative colony size of TSPCs that treated with various concentrations of irisin (0, 2, 5, and 10ng/ml) for 3 d. All data are expressed as mean ± SD (n = 3). One-way ANOVA with a subsequent post hoc Tukey’s test was used for multiple comparisons. *p < 0.05, **p< 0.01 versus the blank group.
Involvement of irisin in tenogenic differentiation of rat TSPCs
TSPCs exhibit spontaneous tenogenic differentiation potentials in vitro (Shin et al. 2020; Liu et al. 2021). It has been recently demonstrated that the erroneous differentiation of TSPCs to non-tenocytes is involved in the pathogenesis of chronic tendinopathy (Lui and Chan 2011). Therefore, promoting differentiation of TSPCs into tenocytes under pathological conditions has been identified as an effective strategy for treatment of tendinopathy. To investigate the role of irisin on tenogenic differentiation of rat TSPCs, the mRNA and protein expression levels of tenogenic markers and extracellular matrix (ECM) markers were assessed by RT-PCR and Western blot. As illustrated in Fig. 2a, irisin could dose-dependently upregulate the mRNA expression of COL1, SCX, and TNMD. Moreover, the results of Western blot showed that the COL1, SCX, and TNMD protein expression levels also increased with irisin treatment in a dose-dependent manner (Fig. 2b–c). These results suggested that irisin could markedly promote tenogenic differentiation to some extent.
Effects of irisin on tenogenic differentiation of rat TSPCs. TSPCs were treated with various concentrations of irisin (0, 2, 5, and 10ng/ml) for 3 d. (a) The mRNA expression levels of extracellular matrix marker (COL1) and tenogenic markers (SCX, TNMD) were evaluated by RT-PCR. (b–c) The protein expression levels of extracellular matrix marker (COL1) and tenogenic markers (SCX, TNMD) were evaluated by Western blot. GAPDH as an internal control. All data are expressed as mean ± SD (n = 3). One-way ANOVA with a subsequent post hoc Tukey’s test was used for multiple comparisons. *p < 0.05, **p< 0.01 versus the blank group. COL1, type I collagen; SCX, scleraxis; TMND, tenomodulin.
Irisin regulated YAP/TAZ expression to promote tenogenic differentiation of rat TSPCs
The role of the Hippo pathway and its downstream effectors, the transcriptional co-activators Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ), on organ regeneration and regenerative medicine has attracted extensive attention of scholars in recent years (Moya and Halder 2019). To further clarify the detailed mechanism of irisin regulation of tenogenic differentiation, the influence of irisin on YAP/TAZ signaling in rat TSPCs was investigated. Protein levels of YAP, TAZ in the total cell lysate, as well as in the nucleus were assessed by Western blot. Results showed that, compared with the control group, the total and nuclear protein expression levels of YAP and TAZ were both significantly upregulated after treating with irisin for 3 d and 7 d (Fig. 3a–b). What’s more, the expression of downstream target genes was also detected by RT-PCR. As shown in Fig. 3c, the mediators of tenogenic induction (CTGF, bFGF) and proliferation promotion (C-MYC) mRNA levels were markedly upregulated by irisin treatment.
Effects of irisin on the expression of YAP/TAZ and the downstream target genes in rat TSPCs. TSPCs were treated with 10ng/ml irisin for various durations (0, 1, 3, and 7 d). (a–b) The protein expression levels of YAP, TAZ in the total cell lysate, as well as in the nucleus were evaluated by Western blot. GAPDH was used as an endogenous control in the cytoplasm, whereas TBP worked as an endogenous control in the nucleus. (c) The mRNA expression levels of CTGF, bFGF, and C-MYC were evaluated by RT-PCR. All data are expressed as mean ± SD (n = 3). One-way ANOVA with a subsequent post hoc Tukey’s test was used for multiple comparisons. *p < 0.05, **p< 0.01 versus the blank group. YAP, Yes-associated protein; TAZ, transcriptional co-activator with PDZ-binding motif; CTGF, connective tissue growth factor; bFGF, basic-fibroblast growth factor.
Previous studies have demonstrated that YAP/TAZ in the nuclear is crucial for the expression of target genes (Mo et al. 2014; Kwon et al. 2021; Ramos and Camargo 2012). Here, the morphological changes of treated TSPCs were observed by the optical microscopy, and the immunofluorescence staining of YAP in TSPCs incubated with 10ng/ml irisin for various times (0, 1d, 3d, 7d) was performed. TSPCs treated with irisin appeared slender and elongated, and the fluorescence intensity of cytoplasmic and nuclear YAP both increased with the duration of irisin treatment, while the enhanced YAP fluorescence signals were mainly observed in the nuclear of TSPCs (Fig. 4). To clarify why the protein expression levels of YAP were upregulated by irisin treatment, we further detected the effects of irisin on YAP mRNA expression levels by RT-PCR. The results demonstrated that the mRNA expression levels of YAP had no significant change after irisin treatment (Figure S2a). Since the overall protein levels of YAP/TAZ are known to be regulated by proteasomal degradation, we further investigated the role of irisin on baseline proteasome activity. Results suggested that irisin treatment reduced proteasome activity (Figure S2b). Together these results indicated that irisin treatment upregulated the protein expression levels of YAP/TAZ and this process was associated with a ubiquitin-proteasome proteolytic pathway.
Effects of irisin on the morphological changes and subcellular positioning of YAP/TAZ in rat TSPCs. TSPCs were treated with 10ng/ml irisin for various durations (0, 1, 3, and 7 d). Optical morphological appearance of TSPCs, bar = 100μm. Immunofluorescence of YAP in TSPCs (red: YAP; blue: DAPI), bar = 50μm.
In order to further ascertain the involvement of YAP/TAZ in the irisin-promoted tenogenic differentiation of rat TSPCs, we subsequently performed the pathway inhibition experiments using verteporfin, the inhibitor of YAP. As shown in Fig. 5a, RT-PCR results revealed that the increments of mRNA expression levels of COL1, SCX, and TNMD caused by irisin were markedly abolished by verteporfin treatment. Meanwhile, the protein expression levels of COL1, SCX, and TNMD showed similar changes following administration of verteporfin according to the Western blot results (Fig. 5b–c). Taken together, these data demonstrated that irisin could promote tenogenic differentiation of rat TSPCs via activating YAP/TAZ.
Effects of verteporfin on the irisin-promoted tenogenic differentiation of rat TSPCs. TSPCs were untreated or treated with 10ng/ml irisin or treated with 5μM verteporfin or treated with both for 3 d. (a) The mRNA expression levels of COL1, SCX, and TNMD were evaluated by RT-PCR. (b–c) The protein expression levels of COL1, SCX, and TNMD were evaluated by Western blot. GAPDH as an internal control. All data are expressed as mean ± SD (n = 3). One-way ANOVA with a subsequent post hoc Tukey’s test was used for multiple comparisons. *p < 0.05, **p< 0.01. VP, verteporfin.
Discussion
Now available treatments of tendinopathy still only control the symptoms due to the poor understanding of tendinopathy pathogenesis. Current evidence has demonstrated that the depletion of stem cell pool and altered TSPCs fate might account for the pathogenesis of tendinopathy (Lui and Chan 2011; Rui et al. 2013). Therefore, stem cell–based therapy has gained more attention in recent years. New therapeutic strategies about stem cell–based therapy for tendon repair and regeneration are emerging all the time.
Many kinds of stem cells, such as embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and mesenchymal stem cells (MSCs), have been proved to be the cell sources for tendon repair due to their potential for differentiating into tenocytes. Many animal studies have verified the active effects of these stem cells on tendon healing (Chen et al. 2009; Xu et al. 2013; Docheva et al. 2015). Tendon-derived stem/progenitor cells (TSPCs) are derived from tendon tissue. Unlike other MSCs, TSPCs express two tendon lineage-related markers scleraxis (SCX) and tendomodulin (TNMD) in vitro, and can differentiate into tendon-like tissues when implanted in vivo (Zhang et al. 2016).
However, there is a risk of ectopic ossification and tumor formation after stem cell transplantation (Harris et al. 2004; Zhang et al. 2008), which may be attributable to the erroneous differentiation of implanted stem cells. Thus, promoting TSPCs to differentiate towards the tenogenic lineage before transplantation might be a good strategy for successful tendon regeneration and repair. Recently, administration of various exogenous factors to promote exogenous tenogenesis has been proven to be effective. Growth differentiation factors (GDFs), connective tissue growth factor (CTGF), platelet-rich plasma (PRP), and biomaterial scaffold engineering are promising factors to induce tenogenic differentiation of TSPCs in vitro (Zhang et al. 2016).
It is well accepted that exercise is an important life style to prevent a variety of disorders (Tu et al. 2020). Current tendinopathy management tends to support exercise-based tendon rehabilitation as the most evidence-based intervention (Malliaras 2017; Rio et al. 2017; Cardoso et al. 2019). However, the potential mechanisms and key mediators of the positive effects of exercise remain unclear. FNDC5/Irisin, as one of novel exercise-induced myokines, might be involved in the exercise-induced protective effects on tendinopathy.
In the present study, we analyzed the effects of irisin on the proliferation and tenogenic differentiation of TSPCs, focusing on the phenotypic changes of TSPCs and the underlying mechanism. Our study firstly identified the TSPCs by the immunophenotype (CD29(+), CD44(+), CD90(+), and CD45(−)), and it was observed that the protein expression levels of FNDC5 in TSPCs contiguously increased in a time-dependent manner over a 7-d period of the tenogenic differentiation. Interestingly, the secretion of irisin in TSPCs presented a decreasing tendency after day 1. Irisin, as the secreted form of FNDC5, is proteolytically cleaved and secreted in response to physical stimulation. It is likely that while the protein expression levels of FNDC5 in TSPCs increased during the tenogenic differentiation, the cleavage of irisin from FNDC5 and secretion from TSPCs was not affected. To evaluate whether irisin could directly regulate the TSPC tenogenic differentiation process, the tenogenic markers and extracellular matrix (ECM) markers were assessed. We found that the mRNA and protein expression levels of COL1, SCX, and TNMD upregulated with increasing irisin concentration. What’s more, in line with previous studies (Würgler-Hauri et al. 2007; Chen et al. 2008), increased CTGF mRNA expression was observed during the irisin-induced TSPC tenogenic differentiation process in our study. Besides, we also found the upregulated mRNA expression of another tenogenic induction gene (bFGF) and proliferation promotion gene (C-MYC) in TSPCs during irisin treatment, which might also contribute to the irisin-induced proliferation and tenogenic differentiation of TSPCs.
The Hippo pathway has been proven to play crucial roles in the regulation of stem cell pluripotency/differentiation (Ramos and Camargo 2012; Mo et al. 2014). The Hippo pathway can be regulated by various factors, such as cellular stresses, mechanical stimuli, and biochemical signals (Kwon et al. 2021). YAP and TAZ are essential transcriptional co-activators and downstream effectors of the Hippo pathway, regulating the expression of target genes related to cell proliferation, tissue homeostasis, and tissue regeneration (Lin et al. 2017). In our study, we found that irisin treatment could upregulate total and nuclear protein levels of YAP/TAZ, and further promoted the expression of downstream target genes (CTGF, bFGF, and C-MYC). Verteporfin, the inhibitor of YAP, was found to reverse the effect of irisin on TSPCs. These results indicated that YAP/TAZ signaling participated in the process of irisin-induced tenogenic differentiation of TSPCs.
However, there are still some limitations in our study. Firstly, we confirmed the positive effect of irisin on TSPC proliferation and tenogenic differentiation in vitro via upregulating YAP/TAZ protein expression. But the effect of irisin on tendon healing in vivo should be further investigated. Secondly, there is a large difference between the circulating irisin concentrations in rat and human, so the role of irisin on human cells should be further evaluated. Last but not the least, in order to explore the precise exercise treatment program for individuals, the release mode of irisin by muscle cells when responding to exercise needs to be further investigated.
Conclusions
In summary, our findings indicated that irisin promoted the proliferation and tenogenic differentiation of rat TSPCs in vitro by activating YAP/TAZ. Therefore, our study provides a novel strategy for tendon repair. Irisin may be a powerful exercise-induced agent for tendinopathy treatment, and YAP/TAZ could be a promising target.
References
Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF (2007) Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13:1219–1227
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468
Cardoso TB, Pizzari T, Kinsella R, Hope D, Cook JL (2019) Current trends in tendinopathy management. Best Pract Res Clin Rheumatol 33:122–140
Chen CH, Cao Y, Wu YF, Bais AJ, Gao JS, Tang JB (2008) Tendon healing in vivo: gene expression and production of multiple growth factors in early tendon healing period. J Hand Surg Am 33:1834–1842
Chen X, Song XH, Yin Z, Zou XH, Wang LL, Hu H, Cao T, Zheng M, Ouyang HW (2009) Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells 27:1276–1287
Docheva D, Müller SA, Majewski M, Evans CH (2015) Biologics for tendon repair. Adv Drug Deliv Rev 84:222–239
Durgam S, Schuster B, Cymerman A, Stewart A, Stewart M (2016) Differential adhesion selection for enrichment of tendon-derived progenitor cells during in vitro culture. Tissue Eng Part C Methods 22:801–808
Gaspar D, Spanoudes K, Holladay C, Pandit A, Zeugolis D (2015) Progress in cell-based therapies for tendon repair. Adv Drug Deliv Rev 84:240–256
Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ (2004) Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res 22:998–1003
Kwon H, Kim J, Jho EH (2021) Role of the Hippo pathway and mechanisms for controlling cellular localization of YAP/TAZ. Febs j.
Lin KC, Park HW, Guan KL (2017) Regulation of the Hippo pathway transcription factor TEAD. Trends Biochem Sci 42:862–872
Liu L, Hindieh J, Leong DJ, Sun HB (2017) Advances of stem cell based-therapeutic approaches for tendon repair. J Orthop Translat 9:69–75
Liu Y-J, Wang H-J, Xue Z-W, Cheang L-H, Tam M-S, Li R-W, Li J-R, Hou H-G, Zheng X-F (2021) Long noncoding RNA H19 accelerates tenogenic differentiation by modulating miR-140-5p/VEGFA signaling. European journal of histochemistry : EJH 65:3297
Lui PP, Chan KM (2011) Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev Rep 7:883–897
Malliaras P (2017) Understanding mechanisms to improve exercise interventions in tendinopathy. Phys Ther Sport 27:50–51
Mo JS, Park HW, Guan KL (2014) The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 15:642–656
Moya IM, Halder G (2019) Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol 20:211–226
Murphy M, Travers M, Gibson W, Chivers P, Debenham J, Docking S, Rio E (2018) Rate of improvement of pain and function in mid-portion Achilles tendinopathy with loading protocols: a systematic review and longitudinal meta-analysis. Sports Med 48:1875–1891
Panati K, Suneetha Y, Narala VR (2016) Irisin/FNDC5--an updated review. Eur Rev Med Pharmacol Sci 20: 689-697.
Rabiee F, Lachinani L, Ghaedi S, Nasr-Esfahani MH, Megraw TL, Ghaedi K (2020) New insights into the cellular activities of Fndc5/Irisin and its signaling pathways. Cell Biosci 10:51
Ramos A, Camargo FD (2012) The Hippo signaling pathway and stem cell biology. Trends Cell Biol 22:339–346
Rio E, van Ark M, Docking S, Moseley GL, Kidgell D, Gaida JE, van den Akker-Scheek I, Zwerver J, Cook J (2017) Isometric contractions are more analgesic than isotonic contractions for patellar tendon pain: an in-season randomized clinical trial. Clin J Sport Med 27:253–259
Rui YF, Lui PP, Li G, Fu SC, Lee YW, Chan KM (2010) Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A 16:1549–1558
Rui YF, Lui PP, Wong YM, Tan Q, Chan KM (2013) Altered fate of tendon-derived stem cells isolated from a failed tendon-healing animal model of tendinopathy. Stem Cells Dev 22:1076–1085
Shin MJ, Shim IK, Kim DM, Choi JH, Lee YN, Jeon IH, Kim H, Park D, Kholinne E, Yang HS, Koh KH (2020) Engineered cell sheets for the effective delivery of adipose-derived stem cells for tendon-to-bone healing. Am J Sports Med 48:3347–3358
Tu T, Peng J, Jiang Y (2020) FNDC5/Irisin: a new protagonist in acute brain injury. Stem Cells Dev 29:533–543
Würgler-Hauri CC, Dourte LM, Baradet TC, Williams GR, Soslowsky LJ (2007) Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg 16:S198–S203
Xu W, Wang Y, Liu E, Sun Y, Luo Z, Xu Z, Liu W, Zhong L, Lv Y, Wang A, Tang Z, Li S, Yang L (2013) Human iPSC-derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model. Tissue Eng Part A 19:2439–2451
Yin Z, Chen X, Chen JL, Shen WL, Hieu Nguyen TM, Gao L, Ouyang HW (2010) The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials 31:2163–2175
Zhang J, Pan T, Liu Y, Wang JH (2010) Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen. J Orthop Res 28:1178–1183
Zhang J, Wang JH (2010) Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord 11:10
Zhang WY, de Almeida PE, Wu JC (2008) Teratoma formation: a tool for monitoring pluripotency in stem cell research. StemBook, In
Zhang X, Lin YC, Rui YF, Xu HL, Chen H, Wang C, Teng GJ (2016) Therapeutic roles of tendon stem/progenitor cells in tendinopathy. Stem Cells Int 2016:4076578
Funding
This work was supported by grants from the Natural Science Foundation of Zhejiang Province (LGF20H310004) and the National Natural Science Foundation of China (81901122).
Author information
Authors and Affiliations
Corresponding authors
Supplementary information

Fig. S1
Effects of tenogenic differentiation induction medium on irisin secretion in rat TSPCs. TSPCs were cultured in the tenogenic differentiation induction medium for 7 d, and the medium was changed every day. The irisin concentrations of cell culture supernates at day 1, 3, and 7 were evaluated by ELISA. *p < 0.05 versus the blank group. (PNG 19 kb)

Fig. S2
Effects of irisin on the mRNA expression levels of YAP and proteasomal degradation in rat TSPCs. TSPCs were treated with 10ng/ml irisin for various durations (0, 1, 3, and 7 d). (a) The mRNA expression levels of YAP were evaluated by RT-PCR. (b) The chymotrypsin-like activity of the 26S proteasome was evaluated via cell-based luminescent assay. *p < 0.05 versus the blank group. (PNG 387 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, L., Chen, Z., Geng, T. et al. Irisin promotes the proliferation and tenogenic differentiation of rat tendon-derived stem/progenitor cells via activating YAP/TAZ. In Vitro Cell.Dev.Biol.-Animal 58, 658–668 (2022). https://doi.org/10.1007/s11626-022-00699-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-022-00699-2