Abstract
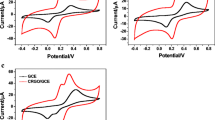
A simple and green method was introduced to fabricate the electrochemical sensor based on the electrochemically reduced graphene oxide (ERGO) modified electrode. It was found that the ERGO modified electrode exhibited an excellent catalytic activity toward the oxidation of dihydroxybenzenes isomers. Under the optimum conditions, in the simultaneous determination of the dihydroxybenzene isomers, the currents were linear with the concentrations of the isomers from 5 to 550 μmol/L for hydroquinone (HQ), from 6 to 400 μmol/L for catechol (CT) and from 5 to 350 μmol/L for resorcinol (RS), respectively. In addition, the proposed sensor has good stability and reproducibility. The developed method has been applied to the simultaneous determination of dihydroxybenzene isomers in real samples with a satisfactory recovery from 98% to 103%.
Similar content being viewed by others
References
Xie T, Liu Q, Shi Y, et al. Simultaneous Determination of Positional Isomers of Benzenediols by Capillary Zone Electrophoresis with Square Wave Amperometric Detection[J]. J.Chromatogr. A, 2006, 1109(2): 317–321
Valentini F, Ciambella E, Conte V, et al. Highly Selective Detection of Epinephrine at Oxidized Single-Wall Carbon Nanohorns Modified Screen Printed Electrodes (SPEs)[J]. Biosens. Bioelectron, 2014, 59(13): 94–98
Prathap M U A, Satpati B, Srivastava R. Facile Preparation of Polyaniline/MnO2 Nanofibers and Its Electrochemical Application in the Simultaneous Determination of Catechol, Hydroquinone, and Resorcinol [J]. Sens. Actuators, B, 2013, 186(6): 67–77
Gan T, Sun J, Wu W, et al. Electrochemical Determination of Dopamine Using a Mesoporous MnO2/Polypyrrole-Modified Electrode [J]. Nanosci. Nanotech. Lett., 2013, 5(6): 673–676
Tang W, Zhang M, Li W, et al. An Electrochemical Sensor Based on Polyaniline for Monitoring Hydroquinone and Its Damage on DNA[J]. Talanta., 2014, 127: 262–268
Gumpu M B, Nesakumar N, Sethuraman S, et al. Development of Electrochemical Biosensor with Ceria-PANI Core-Shell Nano-Interface for the Detection of Histamine[J]. Sens. Actuators, B, 2014, 199(6): 330–338
Stoller M D, Park S, Zhu Y, et al. Graphene-Based Ultracapacitors[J]. Nano Lett., 2008, 8(10): 3498–3502
Morozov S V, Novoselov K S, Katsnelson M I, et al. Giant Intrinsic Carrier Mobilities in Graphene and Its Bilayer[J]. Phys. Rev. Lett., 2008, 100(1): 016602–016605
Gao P, Chen Y J. Reduced Graphene Oxide/Ni1-x CoxAl-Layered Double Hydroxide Composites: Preparation and High Supercapacitor Performance[J]. Dalton T., 2014, 43(30): 11667–11675
Xiong G, Meng C, Reifenberger R G, et al. A Review of Graphene-Based Electrochemical Microsupercapacitors[J]. Electroanal., 2014, 26(1): 30–51
Wang H, Kakade B A, Tamaki T, et al. Synthesis of 3D Graphite Oxide-Exfoliated Carbon Nanotube Carbon Composite and Its Application as Catalyst Support for Fuel Cells[J]. J. Power. Sources., 2014, 260(5): 338–348
Guo H L, Wang X F, Qian Q Y, et al. A Green Approach to the Synthesis of Graphene Nanosheets[J]. ACS Nano., 2009, 3(9): 2653–2659
Hummers Jr W S, Offeman R E. Preparation of Graphitic Oxide[J]. J. Am. Chem. Soc., 1958, 80(6): 1339–1339
Unnikrishnan B, Palanisamy S, Chen S M. A Simple Electrochemical Approach to Fabricate a Glucose Biosensor Based on Graphene-Glucose Oxidase Biocomposite[J]. Biosens. Bioelectron., 2013, 39(1): 70–75
Li S, Deng D H, Pang H, et al. Preparation of Electrochemically Reduced Graphene Oxide-Modified Electrode and Its Application for Determination of P-aminophenol[J]. J. Solid State Electrochem., 2012, 16(9): 2883–2889
Raj M A, John S A. Fabrication of Electrochemically Reduced Graphene Oxide Films on Glassy Carbon Electrode by Self-Assembly Method and Their Electro-Catalytic Application[J]. J. Phys. Chem. C, 2013, 117(8): 4326–4335
Author information
Authors and Affiliations
Corresponding authors
Additional information
Funded by the National Natural Science Foundation of China (No. 51273155) and the Fundamental Research Funds for the Central Universities (No. 2014-Ia-030)
Rights and permissions
About this article
Cite this article
Wang, M., Li, X., Liu, J. et al. Electrochemically reduced graphene oxide as modified electrode material for determination of dihydroxybenzenes. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 32, 1220–1224 (2017). https://doi.org/10.1007/s11595-017-1734-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-017-1734-3