Abstract
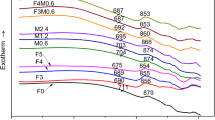
Sm2O3 containing zinc-borosilicate glass and glass ceramics were prepared by melt quenching method, and the effect of Sm2O3 and micro-crystallization on the chemical stability of borosilicate glass was explored. DTA analysis showed that the endothermic peak and exothermic peak of basic glass changed from 635 °C and 834 °C to 630 °C and 828 °C respectively as a result of the doping of Sm2O3. XRD analysis showed the promoting effect of Sm2O3 on crystallization ability of this glass. The cumulative mass loss of base glass, Sm2O3 containing glass, glass ceramic and Sm2O3 containing glass ceramic was 0.289, 0.253, 0.329, 0.269 mg/ mm2 respectively after 26 days corrosion in alkali solution, and 1.293, 1.290, 0.999, 1.040 mg/mm2 respectively in acidic erosion medium. Micro-crystallization decreased and improved the alkali and acid resistance of borosilicate glass respectively, the addition of Sm2O3 increased the alkali resistance of base glass and glass ceramics, and the slight effect of Sm2O3 on the acid resistance of borosilicate glass was also observed.
Similar content being viewed by others
References
He Feng, Fang Yu, Liu Jia, et al. Effect of B2O3 on Structure and Properties of Borosilicate Glass [J]. Journal of Wuhan University of Technology, 2012, 34(2):1–4 (in Chinese)
Christopher W. Sinton, William C. LaCourse. Experimental Survey of the Chemical Durability of Commercial Soda-Lime-Silicate Glasses [J]. Materials Research Bulletin, 2001, 36(13–14): 2 471–2 479
Carmona N, Villegas MA, Fernandez Navarro JM. Corrosion Behaviour of R2O-CaO-SiO2 Glasses Submitted to Accelerated Weathering [J]. Journal of Nuclear Material, 2005, 25(6): 903–910
Sterpenich J, Libourel G. Water Diffusion in Silicate Glasses Under Natural Weathering Conditions: Evidence from Buried Medieval Stained Glasses [J]. Journal of Non-Crystalline Solids, 2006, 352(50–51): 5 446–5 451
Tournie A, Ricciardi P, Colomban Ph. Glass Corrosion Mechanisms: A Multiscale Analysis [J]. Solid State Ionics, 2008, 179(38): 2 142–2 154
Bois L, Barre N, Guillope S, et al. Dissolution of Lanthanide Alumino-Silicate Oxynitrinde Glasses [J]. Journal of Nuclear Material, 2000, 277: 57–66
Bois L, Guittet MJ, Barre N, et al. Aqueous Alteration of Lanthanum Alumino-Silicate Glasses [J]. Journal of Non-Crystalline Solids, 2000, 276(1–3):181–194
Leturcq G, Berger G, Advoccat T, et al. Initial and Long-Term Dissolution Rates of Aluminosilicate Glasses Enriched with Ti, Zr and Nd [J]. Chemical Geology, 1999, 160(1–2): 39–62
Wang MT, Cheng JS, Liu QM, et al. The Effect of Light Rare Earths on the Chemical Durability and Weathering of Na2O-CaO-SiO2 Glasses [J]. Journal of Nuclear Materials, 2010, 400(2): 107–111
Wang MT, Li M, Cheng JS, et al. The Role of Gd2O3 and Y2O3 in Corrosion of Soda Lime Silicate Glass [J]. Journal of Nuclear Materials, 2013, 433(1–3): 287–296
Wang MT, Li M, Cheng JS, et al. Corrosion of Soda Lime Silicate Glasses Co-Doped with Gd2O3 and Y2O3 [J]. Journal of Nuclear Materials, 2013, 444(1–3): 247–251
Dong W, Lu JS. Effect of Rare Earth Doping on Microstructure and Optical Properties of Li2O-Al2O3-SiO2 Glass-Ceramics [J]. Transactions of Materials and Heat Treatment, 2011, 32(8): 19–23 (in Chinese)
Song SF, Wen ZY, Liu Y, et al. Influence of Dopants on the Crystallization of Borosilicate Glass [J]. Ceramics International, 2009, 35(8): 3 037–3 042
Zhang XP, Meng XF, Shan XB, et al. Glass Forming Ability of Samarium Borosilicate Glass [J]. The Chinese Journal of Process Engineering, 2006, 6(1): 120–123 (in Chinese)
El-Okr M, Ibrahem M, Farouk M. Structure and Properties of Rare-Earth-Doped Glassy Systems [J]. Journal of Physics and Chemistry of Solids, 2008, 69(10): 2 564–2 567
Wang MT, Cheng JS, Li M, et al. Structure and Properties of Soda Lime Silicate Glass Doped with Rare Earth [J]. Physica B: Condensed Matter, 2011, 406(2): 187–191
Wang MT, Cheng JS, Li M, et al. Raman Spectra of Soda Lime Silicate Glass Doped with Rare Earth [J]. Physica B: Condensed Matter, 2011, 406(20): 3 865–3 869
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the National Natural Science Foundation of China (51025416, 51202104 and 51362019), the Natural Science Foundation of the Inner Mongolia Autonomous Region (2012MS0807), the Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region, the Talent Incubation Funding of School of Materials and Metallurgy (2014CY012) and the Talent Incubation Funding of School of Materials and Metallurgy(2014CY012)
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, M., Li, M. et al. The effect of Sm2O3 on the chemical stability of borosilicate glass and glass ceramics. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 29, 692–697 (2014). https://doi.org/10.1007/s11595-014-0982-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-014-0982-8