Abstract
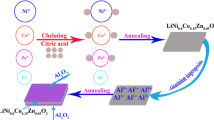
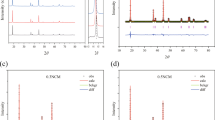
High-nickel cathode material has high specific capacity, but the rapid capacity decay restricts its application. Hereby, three conductive oxides including nano-antimony tin oxide (Sb2SnO5, ATO), zinc stannate (Zn2SnO4, ZTO), and antimony-doped zinc stannate (Sb-doped ZTO, AZTO) with high mobility and low resistivity were used as coating materials separately to improve the rate capability and cycling stability of LiNi0.8Co0.1Mn0.1O2 (NCM811). The conductive oxide was uniformly coated on the surface of NCM811 with the thickness of about 4 nm. The AZTO-coated NCM811 shows the superior rate capability with the initial discharge capacities of 201.35, 197.86, 190.80, and 182.31 mAh g−1 at 0.1, 0.2, 0.5, and 1 C, respectively. The ZTO-coated NCM811 has the best cycling stability with the capacity retention of 85.93% after 100 cycles, while that of the pristine NCM811 is 66.75%. The conductive oxides improve the electronic/ionic conductivity and block the reaction between cathode material and electrolyte, reduce the electrochemical impedance and the side reaction, and further increase the rate capability and the reversibility of the materials.








Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
De Biasi L, Schwarz B, Brezesinski T, Hartmann P, Janek J, Ehrenberg H (2019) Chemical, structural, and electronic aspects of formation and degradation behavior on different length scales of Ni-rich NCM and Li-rich HE-NCM cathode materials in Li-ion batteries. Adv Mater 31:1900985
Ray A, Saruhan B (2021) Application of ionic liquids for batteries and supercapacitors. Mater 14:2942
Xia Y, Zheng J, Wang C, Gu M (2018) Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 49:434–452
Choi JU, Voronina N, Sun YK, Myung ST (2020) Recent progress and perspective of advanced high-energy Co-less Ni-rich cathodes for Li-ion batteries: yesterday, today, and tomorrow. Adv Energy Mater 10:2002027
Ryu HH, Park NY, Yoon DR, Kim UH, Yoon CS, Sun YK (2020) New class of Ni-rich cathode materials Li[NixCoyB1−x−y]O2 for next lithium batteries. Adv Energy Mater 10:2000495
Bi Y, Tao J, Wu Y, Li L, Xu Y, Hu E, Wu B, Hu J, Wang C, Zhang JG, Qi Y, Xiao J (2020) Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 370:1313–1317
Ryu HH, Namkoong B, Kim JH, Belharouak I, Yoon CS, Sun YK (2021) Capacity fading mechanisms in Ni-rich single-crystal NCM cathodes. ACS Energy Lett 6:2726–2734
Choi JW, Aurbach D (2016) Promise and reality of post-lithium-ion batteries with high energy densities. Nat Rev Mater 1:16013
Nair JR, Colo F, Kazzazi A, Moreno M, Bresser D, Lin RY, Bella F, Meligrana G, Fantini S, Simonetti E, Appetecchi GB, Passerini S, Gerbaldi C (2019) Room temperature ionic liquid (RTIL)-based electrolyte cocktails for safe, high working potential Li-based polymer batteries. J Power Sources 412:398–407
Zhu HL, Shen R, Tang YW, Yan XY, Liu J, Song LB, Fan ZQ, Zheng SL, Chen ZY (2020) Sn-doping and Li2SnO3 nano-coating layer co-modified LiNi0.5Co0.2Mn0.3O2 with improved cycle stability at 4.6 V cut-off voltage. Nanomaterials 10:868
Zhao CZ, Zhao Q, Liu X, Zheng J, Stalin S, Zhang Q, Archer LA (2020) Rechargeable lithium metal batteries with an in-built solid-state polymer electrolyte and a high voltage/loading Ni-rich layered cathode. Adv Mater 32:1905629
Park GT, Ryu HH, Park NY, Yoon CS, Sun YK (2019) Tungsten doping for stabilization of Li[Ni0.90Co0.05Mn0.05]O2 cathode for Li-ion battery at high voltage. J Power Sources 442:227242
Ryu HH, Park KJ, Yoon CS, Sun YK (2018) Capacity fading of Ni-rich LiNixCoyMn1-x-yO2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: bulk or surface degradation. Chem Mater 30:1155–1163
Li Q, Dang R, Chen M, Lee Y, Hu Z, Xiao X (2018) Synthesis method for long cycle life lithium-ion cathode material: nickel-rich core-shell LiNi0.8Co0.1Mn0.1O2. ACS Appl Mater Interfaces 10:17850–17860
Dixit M, Markovsky B, Schipper F, Aurbach D, Major DT (2017) Origin of structural degradation during cycling and low thermal stability of Ni-rich layered transition metal-based electrode materials. J Phys Chem C 121:22628–22636
Pekka P, Hubert G (2018) Electrochemical potential window of battery electrolytes: the HOMO–LUMO misconception. Energy Environ Sci 11:2306–2309
Li W, Dolocan A, Oh P (2017) Dynamic behaviour of interphases and its implication on high-energy-density cathode materials in lithium-ion batteries. J Nat Commun 8:14589
Dl V, Jw L (2018) Na-doped layered LiNi0.8Co0.1Mn0.1O2 with improved rate capability and cycling stability. J Solid State Electrochem 22:1165–1173
Abe M, Iba H, Suzuki K, Minamishima H, Hirayama M, Tamura K, Mizuki J, Saito T, Ikuhara Y, Kanno R (2017) Study on the deterioration mechanism of layered rock-salt electrodes using epitaxial thin films – Li (Ni Co, Mn)O2 and their Zr-O surface modified electrodes. J Power Sources 345:108–119
Negi RS, Celik E, Pan R, Staglich R, Senker J (2021) Insights into the positive effect of post-annealing on the electrochemical performance of Al2O3-coated Ni-rich NCM cathodes for lithium-ion batteries. ACS Appl Energy Mater 4:3369–3380
Shao Z, Zhao Z, Zhang Y (2015) Preparation and properties of ZnO, MgO and Al2O3 coated LiNi1/3Co1/3-xMn1/3MxO2 (x = 10%) cathode materials. Mater Technol 30:344–348
Du F, Sun P, Zhou Q, Zeng D, Hu D, Fan Z, Hao Q, Mei C, Xu T, Zheng J (2020) Interlinking primary grains with lithium boron oxide to enhance the stability of LiNi0.8Co0.15Al0.05O2. ACS Appl Mater Interfaces 12:56963–56973
Liu Y, Tang LB, Wei HX, Zhang XH, He ZJ, Li YJ, Zheng JC (2019) Enhancement on structural stability of Ni-rich cathode materials by in-situ fabricating dual-modified layer for lithium-ion batteries. Nano Energy 65:104043
Somo TR, Mabokela TE, Teffu DM, Sekgobela TK, Ramogayana B, Hato MJ, Modibane KD (2021) A comparative review of metal oxide surface coatings on three families of cathode materials for lithium-ion batteries. Coatings 11:744
Sclar H, Maiti S, Leifer N, Vishkin N, Fayena-Greenstein M, Hen M, Grinblat J, Talianker M, Solomatin N, Tiurin O (2021) Electrochemical and thermal behavior of modified Li and Mn-rich cathode materials in battery prototypes: impact of pentasodium aluminate coating and comprehensive understanding of its evolution upon cycling through solid-state, nuclear magnetic resonance A. Adv Energy Sustain Res 2:2000089
Lee S, Li W, Dolocan A, Celio H, Park H, Warner JH, Manthiram A (2021) In-depth analysis of the degradation mechanisms of high-nickel, low/No-cobalt layered oxide cathodes for lithium-ion batteries. Adv Energy Mater 11:2100858
Kim UH, Park JH, Aishova A, Ribas RM, Monteiro RS, Griffiffiffith KJ, Yoon CS, Sun YK (2021) Microstructure engineered Ni-rich layered cathode for electric vehicle batteries. Adv Energy Mater 11:2100884
Becker D, Borner M, Nolle R, Diehl M, Klein S, Rodehorst U, Schmuch R, Winter M, Placke T (2019) Surface modification of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material by tungsten oxide coating for improved electrochemical performance in lithium-ion batteries. ACS Appl Mater Interfaces 11:18404–18414
Medhi R, Li CH, Sang HL, Marquez MD, Lee TR (2019) Uniformly spherical and monodisperse antimony- and zinc-doped tin oxide nanoparticles for optical and electronic applications. ACS Appl Nano Mater 2:6554–6564
Bhat JS, Maddani KI, Karguppikar AM (2006) Influence of Zn doping on electrical and optical properties of multilayered tin oxide thin films. Bull Materials Sci 29:331–337
Vijayalakshmi S, Venkataraj S, Subramanian M, Jayavel R (2008) Physical properties of zinc doped tin oxide films prepared by spray pyrolysis technique. J Phys D Appl Phys 41:035505
Chandra RD, Rao M, Zhang K, Prabhakar RR, Shi C, Zhang J, Mhaisalkar SG, Mathews N (2014) Tuning electrical properties in amorphous zinc tin oxide thin films for solution processed electronics. ACS Appl Mater Interfaces 6:773–777
Jiang Y, Sun W, Xu B, Yan M, Bahlawane N (2011) Unusual enhancement in electrical conductivity of tin oxide thin films with zinc doping. Phys Chem Chem Phys 13:5760–5763
Lu PF, Shen Y, Yu ZY, Zhao L, Li QY, Ma SJ, Han LH, Liu YM (2012) Electronic structure and optical properties of antimony-doped SnO2 from first-principle study. Commun Theor Phys 57:145–150
He X, Feng Z, Zhou L (2020) A synchronously dual-conductive coating towards enhancing the electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode material. J Alloys Compd 852:156966
Liao SY, Huang XW, Rao QS, Li YZ, Hu JQ, Zheng F, Ma ZY, Cui TT, Liu YD, Min YG (2020) A multifunctional MXene additive for enhancing the mechanical and electrochemical performances of the LiNi0.8Co0.1Mn0.1O2 cathode in lithium-ion batteries. J Mater Chem 8:4494–4504
Christiansen TL, Cooper SR, Jensen KM (2020) There’s no place like real-space: elucidating size-dependent atomic structure of nanomaterials using pair distribution function analysis. Nanoscale Adv 2:2234–2254
Xu X, Huo H, Jian J, Wang L, Zhu H, Xu S, He X, Yin G, Du C, Sun X (2019) Radially oriented single-crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium- ion batteries. Adv Energy Mater 9:1803963
Chen ZY, Liu QM, Yan XY, Zhu HL, Liu J, Duan JH, Wang YX (2022) Suppression and mechanism of voltage decay in Sb-doped lithium rich layered oxide cathode materials. J Phys Chem Lett 13:8214–8220
Lee W, Lee D, Kim Y, Choi W, Yoon WS (2020) Enhancing the structural durability of Ni-rich layered materials by post-process: washing and heat-treatment. J Mater Chem A 8:10206–10216
Shi CG, Shen CH, Peng XX, Luo CX, Shen LF, Sheng WJ, Fan JJ, Wang Q, Zhang SJ, Xu BB, Xian JJ, Wei YM, Huang L, Li JT, Sun SG (2019) A special enabler for boosting cyclic life and rate capability of LiNi0.8Co0.1Mn0.1O2: green and simple additive. Nano Energy 65:104084
Hou P, Yin J, Li F, Huang J, Xu X (2019) High-rate and long-life lithium-ion batteries coupling surface-Al3+-enriched LiNi0.7Co0.15Mn0.15O2 cathode with porous Li4Ti5O12 anode. J Chem Eng 378:122057
Yang X, Tang Y, Qu Y, Shang G, Wu J, Zheng J, Lai Y, Li J, Zhang Z (2019) Bifunctional nano-ZrO2 modification of LiNi0.92Co0.08O2 cathode enabling high-energy density lithium ion batteries. J Power Sources 438:226978–226979
Xu M, Chen Z, Li L, Zhu H, Zhao Q, Xu L, Peng N, Gong L (2015) Highly crystalline alumina surface coating from hydrolysis of aluminum isopropoxide on lithium-rich layered oxide. J Power Sources 281:444–454
Ahn J, Yoon S, Jung SG, Yim JH, Cho KY (2017) A conductive thin layer on prepared positive electrodes by vapour reaction printing for high-performance lithium-ion batteries. J Mater Chem 5(40):21214–21222
Tan W, Wang LN, Lu ZG, Yang F, Xu ZH (2022) A hierarchical Si/C nanocomposite of stable conductive network formed through thermal phase separation of asphaltenes for high-performance li-ion batteries. Small 18:35
Zeng XQ, Li M, Abd El-Hady D, Alshitari W, Al-Bogami AS, Lu J, Amine K (2019) Commercialization of lithium battery technologies for electric vehicles. Adv Energy Mater 9:1900161
Jiang J, Li YY, Liu JP, Huang XT, Yuan CZ, Lou XW (2012) Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv Mater 24:5166–5180
Shi Y, Zhang M, Fang C, Meng YS (2018) Urea-based hydrothermal synthesis of LiNi0.5Co0.2Mn0.3O2 cathode material for Li-ion battery. J Power Sources 394:114–121
Hu GR, Zhang MF, Wu LL, Peng ZD, Du K, Cao YB (2017) Effects of Li2SiO3 coating on the performance of LiNi0.5Co0.2Mn0.3O2 cathode material for lithium-ion batteries. J Alloys Compd 690:589–597
Xu C, Xiang W, Wu Z, Qiu L, Ming Y, Yang W, Yue L, Zhang J, Zhong B, Guo X (2021) Dual-site lattice modification regulated cationic ordering for Ni-rich cathode towards boosted structural integrity and cycle stability. Chem Eng J 403:126314
Shen R, Zhu HL, Liu J, Zhu MD, Guo JM, Song LB, Bie XF, Ji Y, Chen ZY (2021) Improved electrochemical performance of Al2O3-coated @ K and Cl dual-doped LiNi0.5Co0.2Mn0.3O2 cathode materials at high cut-off voltage. J Electron Mater 50:5721–5731
Duan H, Fan M, Chen W, Li J, Wang P, Wang W, Shi J, Yin Y, Wan L, Guo Y (2019) Extended electrochemical window of solid electrolytes via heterogeneous multilayered structure for high-voltage lithium metal batteries. Adv Mater 31:1807789
Wu F, Liu N, Chen L, Su Y, Tan G, Bao L, Zhang Q, Lu Y, Wang J, Chen S (2019) Improving the reversibility of the H2–H3 phase transitions for layered Ni-rich oxide cathode towards retarded structural transition and enhanced cycle stability. Nano Energy 59:50–57
Zhong S, Lai M, Yao W, Li Z (2016) Synthesis and electrochemical properties of LiNi0.8CoxMn0.2-xO2 positive-electrode material for lithium-ion batteries. Electrochim Acta 212:343–351
Xie DJ, Li GS, Li Q, Fu CC, Fan JM, Li LP (2016) Improved cycling stability of cobalt-free Li-rich oxides with a stable interface by dual doping. Electrochim Acta 196:505–516
Zhang X, Liu G, Zhou K, Jiao T, Zou Y, Wu Q, Chen X, Yang Y, Zheng J (2021) Enhancing cycle life of nickel-rich LiNi0.9Co0.05Mn0.05O2 via a highly fluorinated electrolyte additive-pentafluoropyridine. Energy Mater 1:100005
Chang H, Wu YR, Han X, Yi TF (2021) Recent developments in advanced anode materials for lithium-ion batteries. Energy Mater 1:100003
Funding
This work was supported by the Scientific Research Fund of Changsha Science and Technology Bureau, Hunan, China (grant number kh2003021); the Scientific Research Foundation of Hunan Provincial Education Department, China (grant number 19B010); the National Natural Science Foundation of Hunan Province, China (grant number 2020JJ4620); and the National Natural Science Foundation of China (grant numbers 51604042 and 51874048).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Md., Zhu, Hl., Guo, Jm. et al. Enhanced rate capability and cycling stability of conductive oxide-coated LiNi0.8Co0.1Mn0.1O2 for lithium-ion batteries. Ionics 29, 1711–1720 (2023). https://doi.org/10.1007/s11581-023-04945-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-04945-9