Abstract
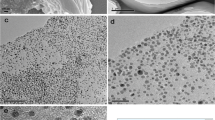
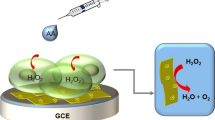
In this paper, we have synthesized the cupric oxide functionalized carbon nanotube-reduced graphene oxide (CuO/CNTs-rGO) nanocomposites by a facile one-pot method. The obtained samples were characterized by transmission electron microscope (TEM), energy-dispersive spectroscopy (EDS), and X-ray powder diffraction (XRD) techniques. To investigate the electrochemical properties, the as-fabricated sensors were tested toward hydrazine detection by cyclic voltammetry (CV) and amperometry methods. The measured results demonstrate that the CuO/CNTs-rGO nanocomposites sensor shows a sensitivity of 4.28 μA·μM−1·cm−2, a linear range of 1.2–430 μM, and detection limit of 0.2 μM toward hydrazine. The enhanced electrocatalytic activity can be attributed to the catalytic properties of CuO and synergistic effect of the ternary nanocomposites. Meanwhile, the as-fabricated CuO/CNTs-rGO sensor also displays good selectivity and long-term stability, which implies its feasibility for practical application.









Similar content being viewed by others
References
Feng Z, Li D, Wang L, Sun Q, Lu P, Xing P, An M (2019) A 3D porous Ni-Zn/RGO catalyst with superaerophobic surface for high-performance hydrazine electrooxidation. J Alloys Compd 788:1240–1245. https://doi.org/10.1016/j.jallcom.2019.03.007
Serov A, Padilla M, Roy AJ, Atanassov P, Sakamoto T, Asazawa K, Tanaka H (2014) Anode catalysts for direct hydrazine fuel cells: from laboratory test to an electric vehicle. Angew Chem Int Ed 53(39):10336–10339. https://doi.org/10.1002/anie.201404734
Hosseini SR, Ghasemi S, Kamali-Rousta M (2017) Preparation of CuO/NiO composite nanofibers by electrospinning and their application for electro-catalytic oxidation of hydrazine. J Power Sources 343:467–476. https://doi.org/10.1016/j.jpowsour.2017.01.083
Hosseini SR, Kamali-Rousta M (2016) Preparation of electro-spun CuO nanoparticle and its application for hydrazine hydrate electro-oxidation. Electrochim Acta 189:45–53. https://doi.org/10.1016/j.electacta.2015.12.070
Ma Y, Wang R, Wang H, Key J, Ji S (2015) Room-temperature synthesis with inert bubble templates to produce “clean” PdCoP alloy nanoparticle networks for enhanced hydrazine electro-oxidation. RSC Adv 5(13):9837–9842. https://doi.org/10.1039/C4RA14423F
Soomro RA, Baloach Q, Tahira A, Ibupoto ZH, Khaskheli GQ, Sirajuddin DVK, Hallam KR, Rajar K, Willander M (2017) Rice-like CuO nanostructures for sensitive electrochemical sensing of hydrazine. Microsyst Technol 23(3):731–738. https://doi.org/10.1007/s00542-015-2726-x
Garrod S, Bollard ME, Nicholls AW, Connor SC, Connelly J, Nicholson JK, Holmes E (2005) Integrated metabonomic analysis of the multiorgan effects of hydrazine toxicity in the rat. Chem Res Toxicol 18(2):115–122. https://doi.org/10.1021/tx0498915
Kumar S, Bhanjana G, Dilbaghi N, Umar A (2015) Zinc oxide nanocones as potential scaffold for the fabrication of ultra-high sensitive hydrazine chemical sensor. Ceram Int 41(2):3101–3108. https://doi.org/10.1016/j.ceramint.2014.10.154
Babu KJ, Zahoor A, Nahm KS, Aziz MA, Vengadesh P, Kumar GG (2016) Manganese dioxide–vulcan carbon@ silver nanocomposites for the application of highly sensitive and selective hydrazine sensors. New J Chem 40(9):7711–7720. https://doi.org/10.1039/C6NJ00268D
Zhao Z, Sun Y, Li P, Zhang W, Lian K, Hu J, Chen Y (2016) Preparation and characterization of AuNPs/CNTs-ErGO electrochemical sensors for highly sensitive detection of hydrazine. Talanta 158:283–291. https://doi.org/10.1016/j.talanta.2016.05.065
Golabi SM, Zare H (1999) Electrocatalytic oxidation of hydrazine at glassy carbon electrode modified with electrodeposited film derived from caffeic acid. Electroanal 11(17):1293–1300. https://doi.org/10.1002/(SICI)1521-4109(199911)11:17<1293::AID-ELAN1293>3.0.CO;2-2
Fang B, Zhang C, Zhang W, Wang G (2009) A novel hydrazine electrochemical sensor based on a carbon nanotube-wired ZnO nanoflower-modified electrode. Electrochim Acta 55(1):178–182. https://doi.org/10.1016/j.electacta.2009.08.036
Gilbert R, Rioux R, Saheb SE (1984) Ion chromatographic determination of morpholine and cyclohexylamine in aqueous solutions containing ammonia and hydrazine. Anal Chem 56(1):106–109. https://doi.org/10.1021/ac00265a029
Guerra SV, Kubota LT, Xavier CR, NAKAGAKI S (1999) Experimental optimization of selective hydrazine detection in flow injection analysis using a carbon paste electrode modified with copper porphyrin occluded into zeolite cavity. Anal Sci 15(12):1231–1234. https://doi.org/10.2116/analsci.15.1231
Umar A, Rahman MM, Kim SH, Hahn YB (2008) Zinc oxide nanonail based chemical sensor for hydrazine detection. Chem Commun 2:166–168. https://doi.org/10.1039/B711215G
Meenakshi S, Kaladevi G, Pandian K, Wilson P (2018) Cobalt phthalocyanine tagged graphene nanoflakes for enhanced electrocatalytic detection of N-acetylcysteine by amperometry method. Ionics 24:2807–2819. https://doi.org/10.1007/s11581-017-2410-5
Zheng L, Song JF (2009) Curcumin multi-wall carbon nanotubes modified glassy carbon electrode and its electrocatalytic activity towards oxidation of hydrazine. Sensors Actuators B Chem 135(2):650–655. https://doi.org/10.1016/j.snb.2008.09.035
Hu J, Zhao Z, Zhang J, Li G, Li P, Zhang W, Lian K (2017) Synthesis of palladium nanoparticle modified reduced graphene oxide and multi-walled carbon nanotube hybrid structures for electrochemical applications. Appl Surf Sci 396:523–529. https://doi.org/10.1016/j.apsusc.2016.10.187
Zhao Z, Sun Y, Li P, Sang S, Zhang W, Hu J, Lian K (2014) A sensitive hydrazine electrochemical sensor based on zinc oxide nano-wires. J Electrochem Soc 161(6):B157–B162. https://doi.org/10.1149/2.095406jes
Dai G, Xie J, Li C, Liu S (2017) Flower-like Co3O4/graphitic carbon nitride nanocomposite based electrochemical sensor and its highly sensitive electrocatalysis of hydrazine. J Alloys Compd 727:43–51. https://doi.org/10.1016/j.jallcom.2017.08.100
Zhou T, Lu P, Zhang Z, Wang Q, Umar A (2016) Perforated Co3O4 nanoneedles assembled in chrysanthemum-like Co3O4 structures for ultra-high sensitive hydrazine chemical sensor. Sensors Actuators B Chem 235:457–465. https://doi.org/10.1016/j.snb.2016.05.075
Zhao Z, Zhang J, Wang W, Sun Y, Li P, Hu J, Chen L, Gong W (2019) Synthesis and electrochemical properties of Co3O4-rGO/CNTs composites towards highly sensitive nitrite detection. Appl Surf Sci 485:274–282. https://doi.org/10.1016/j.apsusc.2019.04.202
Rahman MM, Alfonso VG, Fabregat-Santiago F, Bisquert J, Asiri AM, Alshehri AA, Albar HA (2017) Hydrazine sensors development based on a glassy carbon electrode modified with a nanostructured TiO2 films by electrochemical approach. Microchim Acta 184(7):2123–2129. https://doi.org/10.1007/s00604-017-2228-x
Li Y, Cheng C, Yang Y, Dun X, Gao J, Jin XJ (2019) A novel electrochemical sensor based on CuO/H-C3N4/rGO nanocomposite for efficient electrochemical sensing nitrite. J Alloys Compd 798:764–772. https://doi.org/10.1016/j.jallcom.2019.05.137
Zhang J, Chen L, Yang K (2019) In situ synthesis of CuO nanoparticles decorated hierarchical Ce-metal-organic framework nanocomposite for an ultrasensitive non-enzymatic glucose sensor. Ionics 1-11. https://doi.org/10.1007/s11581-019-02996-5
Vasuki K, Siva G, Balasubramani A, Pannipara M, Al-Sehemi AG, Xia Y, Fang R, Yoo DJ, Kumar TR, Ramachandran R, Gnana kumar G (2019) Surfactant and binder free hierarchical NCNPs@CuO nanostructures on ITO for the cost effective enzyme-free glucose sensor applications. Appl Phys A-Mater 125:384. https://doi.org/10.1007/s00339-019-2652-3, 312
Zhang X, Gu A, Wang G, Wang W, Wu H, Fang B (2009) Seed-mediated preparation of CuO nanoflowers and their application as hydrazine sensor. Chem Lett 38(5):466–467. https://doi.org/10.1246/cl.2009.466
Raj kumar T, Yoo DJ, Kim AR, Gnana kumar G (2019) Green synthesis of Pt-Pd bimetallic nanoparticle decorated reduced graphene oxide and its robust catalytic activity for efficient ethylene glycol electrooxidation. New J Chem 42(17):14386–14393. https://doi.org/10.1039/C8NJ02782J
Amala G, Saravanan J, Yoo DJ, Kim AR, Gnana kumar G (2017) An environmentally benign one pot green synthesis of reduced graphene oxide based composites for the enzyme free electrochemical detection of hydrogen peroxide. New J Chem 41(10): 4022-4030. https://doi.org/10.1039/C6NJ04030F
Ramachandran K, Babu K, Kumar GG, Kim AR, Yoo DJ (2015) One-pot synthesis of graphene supported CuO nanorods for the electrochemical hydrazine sensor applications. Sci Adv Mater 7(2):329–336. https://doi.org/10.1166/sam.2015.2025
Wang L, Meng T, Jia H, Feng Y, Gong T, Wang H, Zhang Y (2019) Electrochemical study of hydrazine oxidation by leaf-shaped copper oxide loaded on highly ordered mesoporous carbon composite. J Colloid Interface Sci 549:98–104. https://doi.org/10.1016/j.jcis.2019.04.063
Mani V, Dinesh B, Chen SM, Saraswathi R (2014) Direct electrochemistry of myoglobin at reduced graphene oxide-multiwalled carbon nanotubes-platinum nanoparticles nanocomposite and biosensing towards hydrogen peroxide and nitrite. Biosens Bioelectron 53:420–427. https://doi.org/10.1016/j.bios.2013.09.075
Hummers WS Jr, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339–1339. https://doi.org/10.1021/ja01539a017
Lu LQ, Wang Y (2012) Facile synthesis of graphene-supported shuttle-and urchin-like CuO for high and fast Li-ion storage. Electrochem Commun 14(1):82–85. https://doi.org/10.1016/j.elecom.2011.11.010
Zhao B, Liu P, Zhuang H, Jiao Z, Fang T, Xu W, Lu B, Jiang Y (2013) Hierarchical self-assembly of microscale leaf-like CuO on graphene sheets for high-performance electrochemical capacitors. J Mater Chem A 1(2):367–373. https://doi.org/10.1039/C2TA00084A
Mehta SK, Umar A (2011) Highly sensitive hydrazine chemical sensor based on mono-dispersed rapidly synthesized PEG-coated ZnS nanoparticles. Talanta 85(5):2411–2416. https://doi.org/10.1016/j.talanta.2011.07.089
Hu J, Zhao Z, Sun Y, Wang Y, Li P, Zhang W, Lian K (2016) Controllable synthesis of branched hierarchical ZnO nanorod arrays for highly sensitive hydrazine detection. Appl Surf Sci 364:434–441. https://doi.org/10.1016/j.apsusc.2015.12.165
Guo Z, Seol ML, Kim MS, Ahn JH, Choi YK, Liu JH, Huang XJ (2012) Hollow CuO nanospheres uniformly anchored on porous Si nanowires: preparation and their potential use as electrochemical sensors. Nanoscale 4(23):7525–7531. https://doi.org/10.1039/C2NR32556J
Teymoori N, Raoof JB, Khalilzadeh MA, Ojani R (2018) An electrochemical sensor based on CuO nanoparticle for simultaneous determination of hydrazine and bisphenol A. J Iran Chem Soc15(10):2271-2279. https://doi.org/10.1007/s13738-018-1416-x
Karim-Nezhad G, Jafarloo R, Dorraji PS (2009) Copper (hydr) oxide modified copper electrode for electrocatalytic oxidation of hydrazine in alkaline media. Electrochim Acta 54(24):5721–5726. https://doi.org/10.1016/j.electacta.2009.05.019
Ma Y, Li H, Wang R, Wang H, Lv W, Ji S (2015) Ultrathin willow-like CuO nanoflakes as an efficient catalyst for electro-oxidation of hydrazine. J Power Sources 289:22–25. https://doi.org/10.1016/j.jpowsour.2015.04.151
Rostami S, Azizi SN, Ghasemi S (2017) Simultaneous electrochemical determination of hydrazine and hydroxylamine by CuO doped in ZSM-5 nanoparticles as a new amperometric sensor. New J Chem 41(22):13712–13723. https://doi.org/10.1039/C7NJ02685D
Yin Z, Liu L, Yang Z (2011) An amperometric sensor for hydrazine based on nano-copper oxide modified electrode. J Solid State Electrochem 15(4):821–827. https://doi.org/10.1007/s10008-010-1161-2
Zhao Z, Wang Y, Li P, Sang S, Zhang W, Hu J, Lian K (2015) A highly sensitive electrochemical sensor based on Cu/Cu2O@ carbon nanocomposite structures for hydrazine detection. Anal Methods 7(21):9040–9046. https://doi.org/10.1039/C5AY02122G
Sultana W, Eraiah B, Vasan HN (2012) Efficient polyglycine modified Au electrode for the detection of hydrazine. Anal Methods 4(12):4115–4120. https://doi.org/10.1039/C2AY25869B
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (51205274, 51602121), the Scientific Research Starting Foundation for Professors and Doctors from Huizhou University (2019JB006), and Program for Innovative Research Team of Huizhou University (IRTHZU).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1018 kb)
Rights and permissions
About this article
Cite this article
Zhao, Z., Wang, W., Tang, W. et al. Synthesis and electrochemistry performance of CuO-functionalized CNTs-rGO nanocomposites for highly sensitive hydrazine detection. Ionics 26, 2599–2609 (2020). https://doi.org/10.1007/s11581-019-03305-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03305-w