Abstract
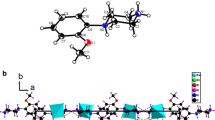
The crystallization of (C3H7N6)Cl•0.5H2O is made by slow evaporation at room temperature. It was found to crystallize in the monoclinic system, C2/m space group, with the following lattice parameters: a = 12.4124 (5) Å, b = 17.6339 (7) Å, c = 7.1193 (3) Å, β = 115.057 (2)°, Z = 4, and V = 1411.61 (10) Å3. The cohesion in (C3H7N6)Cl•0.5H2O is provided by three types of hydrogen bonds, O–H…Cl, N–H…O, N–H…Cl, and N–H… N. Furthermore, the room temperature IR and Raman spectra of the title compound were recorded and analyzed on the basis of literature data. The optical study was also investigated by UV-Vis absorption. The differential scanning calorimetric (DSC) and dielectric study of this compound has been measured. The percentages of hydrogen bonding interactions are analyzed by fingerprint plots of Hirshfeld surface.















Similar content being viewed by others
References
Fernandez-Liencres MP, Navarro A, Lopez-Gonzalez JJ, Fernandez-Gomez M, Tomkinson J, Kearley GJ (2001) Measurement and ab initio modeling of the inelastic neutron scattering of solid melamine. Chem Phys 266:1–17
Ouahab L (1997) Organic/Inorganic Supramolecular Assemblies and Synergy between Physical Properties. Chem Mater 9:1909–1926
Ishihara T, Takahashi J, Goto T (1990) Optical properties due to electronic transitions in two-dimensional semiconductors (CnH2n+1NH3)2PbI4. Phys Rev B 42:11099–11107
Lassoued MS, Abdelbaky MSM, Ben Soltan W, lassoued A, Ammar S, Gadri A, Ben Salah A, Garcia Granda S (2018) Structure characterization, photoluminescence and dielectric properties of a new hybrid compound containing chlorate anions of zincate (II). J Mol Struct 1158:221–228
Takada J, Awaji H, Koshioka M, Nakajima A, Nevin WA (1992) Organic–inorganic multilayers: a new concept of optoelectronic material. Appl Phys Lett 61:2184–2186
Mitzi DB, Field CA, Schlesinger Z, Laibowitz RB Transport(1995) Transport, optical, and magnetic properties of the conducting halide perovskite CH3NH3SnI3. J Solid State Chem 114:159–163
Gomez-Romero P, Chojak M, Cuentas-Gallegos K, Asensio JA, Kulesza PJ, Casan-Pastor N, Lira-Cantu M (2003) Hybrid organic–inorganic nanocomposite materials for application in solid state electrochemical supercapacitors. Electrochem Commun 5:149–153
Baklouti Y, chaari N, Feki H, Chniba-Boudjada N, Zouari F (2015) Crystal structure, vibrational studies, optical properties and DFT calculations of 2-amino-5-diethyl-aminopentanium tetrachlorocadmate (II). Spectrochim Acta A Mol Biomol Spectrosc 136:397–404
Hajlaoui S, Chaabane I, Lhoste J, Bulou A, Guidara K (2016) Structural characterization, vibrational spectroscopy accomplished with DFT calculation, thermal and dielectric behaviors in a new organic-inorganic tertrapropylammonium aquapentachlorostannate dihydrate compound. J Alloys Compd 679:302–315
Shapiro A, Landee CP, Turnbull MM, Jornet J, Deumal M, Novoa JJ, Robb MA, Lewis W (2007) Synthesis, structure, and magnetic properties of an antiferromagnetic spin-ladder complex: Bis(2,3-dimethylpyridinium) tetrabromocuprate. J Am Chem Soc 129:952–959
Saparov B, Mitzi DB (2016) Organic–inorganic perovskites: structural versatility for functional materials design. Chem Rev 116:4558–4596
Lee KW, Lee CH, Lee CE (1996) Phase transitions and critical dynamics in (C18H37NH3)2SnCl6. J Chem Phys 104:6964–6966
Wang S, Mitzi DB, Field CA, Guloy A (1995) Synthesis and characterization of [NH2C(I):NH2]3MI5 (M = Sn, Pb): stereochemical activity in divalent in and lead halides containing single .ltbbrac.110.rtbbrac. perovskite sheets. J Am Chem Soc 117:5297–5302
Mitzi DB, Liang K, Wang S (1998) Synthesis and characterization of [NH2C(I)NH2]2ASnI5with A = iodoformamidinium or formamidinium: the chemistry of cyanamide and tin(II) iodide in concentrated aqueous hydriodic acid solutions. Inorg Chem 37:321–327
SHELXS97, Sheldrick GM (1986) Program for crystal structure solution. University of Göttingen, Germany
SHELXL97, Sheldrick GM (1986) Program for crystal structure solution. University of Göttingen, Germany
Brandenburg K, Berndt M (2001) Diamond version 2.1, crystal impact. Bonn
Wolff SK, Grimwood DJ, Mckinnon JJ, Turner MJ, Jayatilaka D, Spackman MA (2012) Crystal Explorer 3.0. University of Western Australia
Zhang J, Kang Y, Wen YH, Li ZJ, Quin YY, Yao YG (2004) Acta Crystallogr E60:462
Marchewka MK, Janczak J, Debrus S, Baran J, Ratajczak H (2003) Crystal structure, vibrational spectra and nonlinear optical properties of tetrakis(2,4,6-triamino-1,3,5-triazin-1-ium) bis(selenate) trihydrate crystal. Solid State Sci 5:643–652
Tanbug R, Kirschbaum K, Pinkerton A (1999) J Chem Crystallogr 29:45–55
Scoponi M, Polo E, Pradella F, Bertolasi V, Carassiti V, Goberti P (1992) J Chem Soc Perkin Trans 2:1127
Bernstein J, Davis RE, Shimoni L, Chang N-L (1995) Patterns in hydrogen bonding: functionality and graph set analysis in crystals. J Angew Chem Int Ed Engl 34:1555–1573
Saber Lassoued M, Abdelbaky MSM, Mendoza Merono R, Gadri A, Ammar S, Salah AB, García-Granda S (2017) Structure, spectroscopic measurement, thermal studies and optical properties of a new hybrid compound of aquapentachloroindoidate(III) complex. J Mol Struct 1142:73–79
Luo YH, Wu GG, Mao SL, Sun BW (2013) Complexation of different metals with a novel N-donor bridging receptor and Hirshfeld surfaces analysis. Inorg Chim Acta 397:1–9
Spackman MA, McKinnon JJ (2002) Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 4:378–392
Larkin PJ, Makowski MP, Colthoup NB (1999) The form of the normal modes of s-triazine: infrared and Raman spectral analysis and ab initio force field calculations. Spectrochim Acta A 55:1011–1020
Wang YL, Mebel AM, Wu CJ, Chen YT, Lin CE, Jiang JC (1997) IR spectroscopy and theoretical vibrational calculation of the melamine molecule. J Chem Soc Faraday Trans 93:3445–3451
Arjunan V, Kalaivani M, Marchewka MK, Mohan S (2013) Structural and vibrational spectral investigations of melaminium maleate monohydrate by FTIR, FT-Raman and quantum chemical calculations. Spectrochim Acta A Mol Biomol Spectrosc 107:90–101
Krishnan P, Gayathri K, Rajakumar PR, Jayaramakrishnan V, Gunasekaran S, Anbalagan G (2014) Studies on crystal growth, vibrational, optical, thermal and dielectric properties of new organic nonlinear optical crystal: Bis (2,3-dimethoxy-10-oxostrychnidinium) phthalate nonahydrate single crystal. Spectrochim Acta A Mol Biomol Spectrosc 131:114–124
Meier RJ, Tiller A, Vanhommerig SAM (1995) Molecular modeling of melamine-formaldehyde resins. 2. Vibrational spectra of methylolmelamines and bridged methylolmelamines. J Phys Chem 99:5457–5464
Jones WJ, Orville-Thomas WJ (1959) The infra-red spectrum and structure of melamine. Trans Faraday Soc 55:203
Larkin PJ, Makowski MP, Colthup NB, Food LA (1998) Vibrational analysis of some important group frequencies of melamine derivatives containing methoxymethyl, and carbamate substituents: mechanical coupling of substituent vibrations with triazine ring modes. Vib Spectrosc 17:53–72
Jones WJ, Orville-Thomas WJ (1959) The infra-red spectrum and structure of dicyandiamide. Trans Faraday Soc 55:193
Ben Rhaiem A, Guidara K, Gargouri M, Daoud A (2005) Electrical properties and equivalent circuit of trimethylammonium monobromodichloromercurate. J Alloys Compd 392:68–71
Venkaterwarlu P, Laha A, Krupanidhi SB (2005) AC properties of laser ablated La-modified lead titanate thin films. Thin Solid Films 474:1–9
Tareev B (1975) Physics of dielectric materials. Mir Publishers, Moscow
Kurien S, Mathew J, Sebastian S, Potty SN, George KC (2006) Dielectric behavior and ac electrical conductivity of nanocrystalline nickel aluminate. Mater Chem Phys 98:470–476
Anantha PS, Harihanan K (2005) Conductivity analysis and dielectric relaxation behaviour of NaNO3–Al2O3 composites. Mater Sci Eng B 121:12–19
Das PS, Chakraborty PK, Behera B, Choudhary RNP (2007) Electrical properties of Li2BiV5O15 ceramics. Phys B 395:98–103
Behera B, Nayak P, Choudhary RNP (2008) Structural and impedance properties of KBa2V5O15 ceramics. Mater Res Bull 43:401–410
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Supplementary material
Supplementary crystallographic data for this article in CIF format are available as Electronic Supplementary Publication from Cambridge Crystallographic Data Centre (CCDC 1494324). This data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Rood, Cambridge CB2 1EZ, UK (Fax: (international): +441,223/336033; e-mail:deposit@ccdc.cam.ac.uk).
Rights and permissions
About this article
Cite this article
Mesbeh, R., Hamdi, B. & Zouari, R. Synthesis, crystal structure, physicochemical characterization, and dielectric properties of a new organic chloride salt, (C3H7N6)Cl•0.5H2O. Ionics 25, 6147–6160 (2019). https://doi.org/10.1007/s11581-019-03092-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03092-4