Abstract
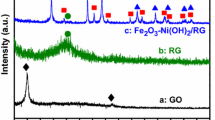
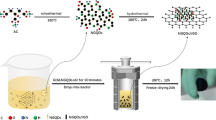
Amorphous Ni (OH)2 nanobox-like materials were synthesized using Cu2O as template. Ni (OH)2/reduced graphene oxide (Ni (OH)2/rGO) composites were prepared by a simple hydrothermal method. The results obtained from field emission scanning electron microscopy and transmission electron microscopy indicated that the Ni (OH)2 nanobox structure broke into small pieces of 50–100 nm under high temperature and high pressure during the hydrothermal process, and successfully covered the entire graphene surface. Electrochemical studies showed that a specific capacitance of 626.84 F g−1 was obtained at a current density of 1 A g−1. When the current density increased from 1 to 5 A g−1, the capacitance retention rate reached 50%. After 2000 consecutive charge–discharge cycles, the capacitance of Ni (OH)2/rGO composite decreased 30% of initial capacitance compared to 55% for pure Ni (OH)2. The rational design, interesting structure, and ideal electrochemical performance of this graphene-based composite indicate its potential application in high-energy storage systems.








Similar content being viewed by others
References
Béguin F, Presser V, Balducci A, Frackowiak E (2014) Carbons and electrolytes for advanced supercapacitors. Adv Mater 26(14):2219–2251
Huang L, Chen D, Ding Y, Wang ZL, Zeng Z, Liu M (2013) Hybrid composite Ni (OH)2@NiCo2O4 grown on carbon fiber paper for high-performance supercapacitors. ACS Appl Mater Interfaces 5(21):11159–11162
Simon P, Gogotsi Y, Dunn B (2014) Where do batteries end and supercapacitors begin? Science 343(6176):1210–1211
Cui J, Cheng F, Lin J, Yang J, Jiang K, Wen Z, Sun J (2017) High surface area C/SiO2 composites from rice husks as a high-performance anode for lithium ion batteries. Powder Technol 311:1–8
Zhu J, Liu A, Wang D (2017) Study on the synergistic lithium storage performance of Sn/graphene nanocomposites via quantum chemical calculations and experiments. Appl Surf Sci 416:751–756
Zhu J, Zhang X, Zeng C, Liu A, Hu G (2017) Preparation of SnS/graphene nanocomposites from Sn/graphene for superior reversible lithium storage. Mater Lett 209:338–341
Pang H, Li X, Zhao Q, Xue H, Lai WY, Hu Z, Huang W (2017) One-pot synthesis of heterogeneous Co3O4-nanocube/Co (OH)2-nanosheet hybrids for high-performance flexible asymmetric all-solid-state supercapacitors. Nano Energy 35:138–145
Zhang S, Yin B, Wang Z, Feng P (2016) Super long-life all solid-state asymmetric supercapacitor based on NiO nanosheets and α-Fe2O3 nanorods. Chem Eng J 306:193–203
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7(11):845–854
Fan Y, Shao H, Wang J, Liu L, Zhang J, Cao C (2011) Synthesis of foam-like freestanding Co3O4 nanosheets with enhanced electrochemical activities. Chem Commun 47(12):3469–3471
Jeong MG, Kai Z, Cherevko S, Kim WJ, Chung CH (2013) Facile preparation of three-dimensional porous hydrous ruthenium oxide electrode for supercapacitors. J Power Sources 244(4):806–811
Singh AK, Sarkar D, Khan GG, Mandal K (2014) Hydrogenated NiO nanoblock architecture for high performance pseudocapacitor. ACS Appl Mater Interfaces 6(7):4684–4692
Zhu J, He J (2012) Facile synthesis of graphene-wrapped honeycomb MnO2 nanospheres and their application in supercapacitors. ACS Appl Mater Interfaces 4(3):1770–1776
Lu Q, Chen JG, Xiao JQ (2013) Nanostructured electrodes for high-performance pseudocapacitors. Angew Chem 52(7):1882–1889
Umeshbabu E, Rajeshkhanna G, Rao GR (2014) Urchin and sheaf-like NiCo2O4 nanostructures: synthesis and electrochemical energy storage application. Int J Hydrog Energy 39(28):15627–15638
Zhang Y, Feng H, Wu X, Wang L, Zhang A, Xia T, Dong H, Li X, Zhang L (2009) Progress of electrochemical capacitor electrode materials: a review. Int J Hydrog Energy 34(11):4889–4899
Wu H, Gu L, Gao G (2015) Hydrothermal synthesis of ultrathin hexagonal nickel hydroxide nanosheets. 颗粒学报 (Particuology) 22(5):114–118
Zhu Y, Cao C, Tao S, Chu W, Wu Z, Li Y (2014) Ultrathin nickel hydroxide and oxide nanosheets: synthesis, characterizations and excellent supercapacitor performances. Sci Rep 4:5787
Kong L-B, Lang J-W, Liu M, Luo Y-C, Kang L (2009) Facile approach to prepare loose-packed cobalt hydroxide nano-flakes materials for electrochemical capacitors. J Power Sources 194(2):1194–1201
Nam KW, Kim KB (2002) A study on the preparation of NiOx electrode via electrochemical route for supercapacitor applications and their charge storage mechanism. J Electrochem Soc 149(3):A346–A354
Pang SC, Anderson MA (2000) Novel electrode materials for thin-film ultracapacitors: comparison of electrochemical properties of sol-gel-derived and electrodeposited manganese dioxide. J Electrochem Soc 147(2):444–450
Feng L, Zhu Y, Ding H, Ni C (2014) Recent progress in nickel based materials for high performance pseudocapacitor electrodes. J Power Sources 267(3):430–444
Lang JW, Kong LB, Wu WJ, Liu M, Luo YC, Kang L (2009) A facile approach to the preparation of loose-packed Ni (OH)2 nanoflake materials for electrochemical capacitors. J Solid State Electrochem 13(2):333–340
Shi F, Li L, Wang X, Gu C, Tu J (2014) Metal oxide/hydroxide-based materials for supercapacitors. RSC Adv 4(79):41910–41921
Zhang J, Kong LB, Cai JJ, Li H, Luo YC, Kang L (2010) Hierarchically porous nickel hydroxide/mesoporous carbon composite materials for electrochemical capacitors. Microporous Mesoporous Mater 132(1–2):154–162
Mao L, Guan C, Huang X, Ke Q, Zhang Y, Wang J (2016) 3D Graphene-nickel hydroxide hydrogel electrode for high-performance supercapacitor. Electrochim Acta 196:653–660
Xi C, Zhu G, Liu Y, Shen X, Zhu W, Ji Z, Kong L (2018) Belt-like nickel hydroxide carbonate/reduced graphene oxide hybrids: synthesis and performance as supercapacitor electrodes. Colloids Surf A Physicochem Eng Asp 538:748–756
Xiao T, Hu X, Heng B, Chen X, Huang W, Tao W, Wang H, Tang Y, Tan X, Huang X (2013) Ni (OH)2 nanosheets grown on graphene-coated nickel foam for high-performance pseudocapacitors. J Alloys Compd 549(2):147–151
Zhang C, Kuila T, Kim NH, Lee SH, Lee JH (2015) Facile preparation of flower-like NiCo2O4/three dimensional graphene foam hybrid for high performance supercapacitor electrodes. Carbon 89:328–339
Dubal DP, Gund GS, Lokhande CD, Holze R (2013) Decoration of spongelike Ni (OH)2 nanoparticles onto MWCNTs using an easily manipulated chemical protocol for supercapacitors. ACS Appl Mater Interfaces 5(7):2446–2454
Min S, Zhao C, Chen G, Qian X (2014) One-pot hydrothermal synthesis of reduced graphene oxide/Ni (OH)2 films on nickel foam for high performance supercapacitors. Electrochim Acta 115(3):155–164
Yan J, Sun W, Wei T, Zhang Q, Fan Z, Wei F (2012) Fabrication and electrochemical performances of hierarchical porous Ni (OH)2 nanoflakes anchored on graphene sheets. J Mater Chem 22(23):11494–11502
Wang Y, Zhou D, Dan Z, Hou M, Wang C, Xia Y (2013) High performance hybrid supercapacitor based on graphene-supported Ni (OH)2-nanowires and ordered mesoporous carbon CMK-5. J Electrochem Soc 160(1):A98–A104
Wang YX, Hu ZA, Wu HY (2011) Preparation and electrochemical performance of alpha-nickel hydroxide nanowire. Mater Chem Phys 126(3):580–583
Wang H, Casalongue HS, Liang Y, Dai H (2010) Ni (OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J Am Chem Soc 132(21):7472–7477
Wang L, Li X, Guo T, Yan X, Tay BK (2014) Three-dimensional Ni (OH)2 nanoflakes/graphene/nickel foam electrode with high rate capability for supercapacitor applications. Int J Hydrog Energy 39(15):7876–7884
Zang X, Sun C, Dai Z, Yang J, Dong X (2017) Nickel hydroxide nanosheets supported on reduced graphene oxide for high-performance supercapacitors. J Alloys Compd 691:144–150
Ji J, Zhang LL, Ji H, Li Y, Zhao X, Bai X, Fan X, Zhang F, Ruoff RS (2013) Nanoporous Ni (OH)2 thin film on 3D ultrathin-graphite foam for asymmetric supercapacitor. ACS Nano 7(7):6237–6243
Aghazadeh M, Golikand AN, Ghaemi M (2011) Synthesis, characterization, and electrochemical properties of ultrafine β-Ni (OH)2 nanoparticles. Int J Hydrog Energy 36(14):8674–8679
Khaleed AA, Bello A, Dangbegnon JK, Madito MJ, Olaniyan O, Barzegar F, Makgopa K, Oyedotun KO, Mwakikunga BW, Ray SC (2017) Solvothermal synthesis of surfactant free spherical nickel hydroxide/graphene oxide composite for supercapacitor application. J Alloys Compd 721:80–91
Yan J, Fan Z, Wei S, Ning G, Tong W, Qiang Z, Zhang R, Zhi L, Fei W (2012) Advanced asymmetric supercapacitors based on Ni (OH)2/graphene and porous graphene electrodes with high energy density. Adv Funct Mater 22(12):2632–2641
And SD, Munichandraiah N (2008) Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J Phys Chem C 112(11):4406–4417
Zhang H, Cao G, Wang Z, Yang Y, Shi Z, Gu Z (2008) Growth of manganese oxide nanoflowers on vertically-aligned carbon nanotube arrays for high-rate electrochemical capacitive energy storage. Nano Lett 8(9):2664–2668
Chang J, Xu H, Sun J, Gao L (2012) High pseudocapacitance material prepared via in situ growth of Ni (OH)2 nanoflakes on reduced graphene oxide. J Mater Chem 22(22):11146–11150
Yuan B, Zheng X, Zhang C, Lv W, Li B, Yang Q (2014) Assembly of Ni (OH)2-graphene hybrids with a high electrochemical performance by a one-pot hydrothermal method. 新型炭材料 (New Carbon Mater) 29(6):426–431
Ghosh D, Giri S, Mandal A, Das CK (2013) Graphene decorated with Ni (OH)2 and Ag deposited Ni (OH) 2 stacked nanoplate for supercapacitor application. Chem Phys Lett 573(6):41–47
Chai H, Peng X, Liu T, Su X, Jia D, Zhou W (2017) High-performance supercapacitors based on conductive graphene combined with Ni (OH)2 nanoflakes. RSC Adv 7(58):36617–36622
Sun J, Wang J, Li Z, Ou J, Niu L, Wang H, Yang S (2015) Graphene-wrapped Ni (OH)2 hollow spheres as novel electrode material for supercapacitors. J Nanosci Nanotechnol 15(9):7010–7017
Liu H, Zhang J, Xu D, Zhang B, Shi L, Huang L, Tan S (2014) In situ formation of Ni (OH)2 nanoparticle on nitrogen-doped reduced graphene oxide nanosheet for high-performance supercapacitor electrode material. Appl Surf Sci 317:370–377
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, W., Chen, Y., Li, F. et al. Preparation of amorphous detrital Ni (OH)2-reduced graphene oxide composite as electrode material for supercapacitor. Ionics 25, 2401–2409 (2019). https://doi.org/10.1007/s11581-018-2677-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2677-1