Abstract
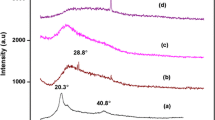
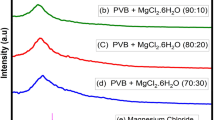
Studies on magnesium ion conducting blend polymer electrolyte (BPE) at room temperature are reported. BPE films of optimized 92.5PVA:7.5PAN ratio in view of its maximum conductivity with various concentrations of magnesium salt—Mg(NO3)2—of different molar mass percentages (0.1, 0.2, 0.3, and 0.4 m.m.%) have been prepared using solution-casting technique with dimethylformamide (DMF) as solvent. The promising characteristic features of these films have been studied. Possible conformational changes in the polymer host 92.5PVA:7.5PAN due to Mg(NO3)2 entrapment have been investigated by FTIR and XRD analysis. The composition 92.5PVA:7.5PAN:0.3 m.m.% Mg(NO3)2 offers maximum electrical conductivity of 1.71 × 10−3 S/cm at room temperature. The σ values follow the Arrhenius equation, and the activation energy for the optimized composition is 0.36 eV. The variations in glass transition temperature have been found using differential scanning calorimeter. Mg2+ ion conduction in the BPE film is confirmed using transference number measurement and cyclic voltammetry. The transport number of the Mg2+ ion is 0.30. Using linear sweep voltammetry, the electrochemical stability window for the highest BPE has been measured. Primary magnesium battery has been constructed using the maximum conducting membrane, and its output characteristics have been studied.












Similar content being viewed by others
References
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Crowther O, West AC (2008) Effect of electrolyte composition on lithium dendrite growth. J Electrochem Soc 155:A806–A811
Matsui M (2011) Study on electrochemically deposited Mg metal. J Power Sources 196:7048–7055
Kim HS, Arthur TS, Allred GD, Zajicek J, Newman JG, Rodnyansky AE, Oliver AG, Boggess WC, Muldoon J (2011) Structure and compatibility of a magnesium electrolyte with a sulphur cathode. Nat Commun 2:427
Wu ID, Chang FC (2007) Determination of the interaction within polyester-based solid polymer electrolyte using FTIR spectroscopy. Polymer 48:989–996
Papke BL, Ratner MA, Shriver DF (1982) Conformation and ion-transport models for the structure and ionic conductivity in complexes of polyethers with alkali metal salts. J Electrochem Soc 129:1694–1701
Kesavan K, Mathew CM, Rajendran S (2014) Lithium ion conduction and ion-polymer interaction in poly (vinyl pyrrolidone) based electrolytes blended with different plasticizers. Chin Chem Lett 25:1428–1434
Hema M, Tamilselvi P, Hirankumar G (2017) Influences of LiCF3SO3 and TiO2 nanofiller on ionic conductivity and mechanical properties of PVA: PVdF blend polymer electrolyte. Ionics 23:2707–2714
Subramania A, Sundaram NK, Kumar GV, Vasudevan T (2006) New polymer electrolyte based on (PVA–PAN) blend for Li-ion battery applications. Ionics 12:175–178
Polu AR, Kumar R, Rhee HW (2015) Magnesium ion conducting solid polymer blend electrolyte based on biodegradable polymers and application in solid-state batteries. Ionics 21:125–132
Ramesh S, Winie T, Arof AK (2007) Investigation of mechanical properties of polyvinyl chloride–polyethylene oxide (PVC–PEO) based polymer electrolytes for lithium polymer cells. Eur Polym J 43:1963–1968
Rajendran S, Sivakumar P (2008) An investigation of PVdF/PVC-based blend electrolytes with EC/PC as plasticizers in lithium battery applications. Phys B Condens Matter 403:509–516
Subramania A, Sundaram NK, Kumar GV (2006) Structural and electrochemical properties of micro-porous polymer blend electrolytes based on PVdF-co-HFP-PAN for Li-ion battery applications. J Power Sources 153:177–182
Mohamad AA, Mohamed NS, Yahya MZA, Othman R, Ramesh S, Alias Y, Arof AK (2003) Ionic conductivity studies of poly (vinyl alcohol) alkaline solid polymer electrolyte and its use in nickel–zinc cells. Solid State Ionics 156:171–177
Kadir MFZ, Majid SR, Arof AK (2010) Plasticized chitosan–PVA blend polymer electrolyte based proton battery. Electrochim Acta 55:1475–1482
Yuan HK, Ren J, Ma XH, Xu ZL (2011) Dehydration of ethyl acetate aqueous solution by pervaporation using PVA/PAN hollow fiber composite membrane. Desalination 280:252–258
Kim DH, Na SK, Park JS, Yoon KJ, Ihm DW (2002) Studies on the preparation of hydrolyzed starch-g-PAN (HSPAN)/PVA blend films––effect of the reaction with epichlorohydrin. Eur Polym J 38:1199–1204
Ma XH, Xu ZL, Liu Y, Sun D (2010) Preparation and characterization of PFSA–PVA–SiO 2/PVA/PAN difunctional hollow fiber composite membranes. J Membr Sci 360:315–322
Sivadevi S, Selvasekarapandian S, Karthikeyan S, Sanjeeviraja C, Nithya H, Iwai Y, Kawamura J (2015) Proton-conducting polymer electrolyte based on PVA-PAN blend doped with ammonium thiocyanate. Ionics 21:1017–1029
Sivadevi S, Selvasekarapandian S, Karthikeyan S, Vijaya N, Kingslin Mary Genova F, Sanjeeviraja C (2013) Structural and AC impedance analysis of blend polymer electrolyte based on PVA and PAN. Int J Sci Res. https://doi.org/10.15373/22778179
Sivadevi S, Selvasekarap S, Karthikeyan S, Vijaya N, Kingslin F, Genova M et al (2013) Proton-conducting polymer electrolyte based on PVA-PAN blend polymer Doped with NH4NO3. Int J Electroactive Mater 1:64–70
Genova FKM, Selvasekarapandian S, Karthikeyan S, Vijaya N, Pradeepa R, Sivadevi S (2015) Study on blend polymer (PVA-PAN) doped with lithium bromide. Polymer Science Series A 57:851–862
Francis KMG, Subramanian S, Shunmugavel K, Naranappa V, Pandian SSM, Nadar SC (2016) Lithium ion-conducting blend polymer electrolyte based on PVA–PAN doped with lithium nitrate. Polym-Plast Technol Eng 55:25–35
Kumar GG, Munichandraiah N (2001) Solid-state rechargeable magnesium cell with poly (vinylidenefluoride)–magnesium triflate gel polymer electrolyte. J Power Sources 102:46–54
Kumar GG, Munichandraiah N (2002) Poly (methylmethacrylate)—magnesium triflate gel polymer electrolyte for solid state magnesium battery application. Electrochim Acta 47:1013–1022
Osman Z, Zainol NH, Samin SM, Chong WG, Isa KM, Othman L et al (2014) Electrochemical impedance spectroscopy studies of magnesium-based polymethylmethacrylate gel polymer electroytes. Electrochimica Acta 131:148–153
Kim JH, Lee YM (2001) Gas permeation properties of poly (amide-6-b-ethylene oxide)–silica hybrid membranes. J Membr Sci 193(2):209–225
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semicrystalline poly (vinyl alcohol) films. Polymer 37:1371–1376
Aji MP, Masturi M, Bijaksana S, Khairurrijal K, Abdullah M (2012) A general formula for ion concentration-dependent electrical conductivities in polymer electrolytes. Am J Appl Sci 9(6):946–954
Ravi M, Song S, Wang J, Wang T, Nadimicherla R (2016) Ionic liquid incorporated biodegradable gel polymer electrolyte for lithium ion battery applications. J Mater Sci Mater Electron 27:1370–1377
Reddy MJ, Chu PP (2002) Effect of Mg 2+ on PEO morphology and conductivity. Solid State Ionics 149(1):115–123
Fattoum A, Arous M, Pedicini R, Carbone A, Charnay C (2015) Conductivity and dielectric relaxation in crosslinked PVA by oxalic and citric acids. Polymer Science Series A 57:321–329
Pandey GP, Agrawal RC, Hashmi J (2011) Magnesium ion-conducting gel polymer electrolytes dispersed with fumed silica for rechargeable magnesium battery application. Solid State Electrochem 15:2253–2264
Rajendran S, Sivakumar M, Subadevi R (2003) Effect of salt concentration in poly (vinyl alcohol)-based solid polymer electrolytes. J Power Sources 124(1):225–230
Abdelrazek EM, Elashmawi IS, Labeeb S (2010) Chitosan filler effects on the experimental characterization, spectroscopic investigation and thermal studies of PVA/PVP blend films. Phys B Condens Matter 405:2021–2027
Pu H, Huang P (2006) Studies on transparent and solid proton conductors based on NH 4 H 2 PO 4 doped poly (vinyl alcohol). Mater Lett 60:1724–1727
Coleman MM, Petcavich RJ (1978) Fourier transform infrared studies on the thermal degradation of polyacrylonitrile. J Polym Sci B Polym Phys 16:821–832
Wahab A, Mahiuddin S (2008) Density, ultrasonic velocity, electrical conductivity, viscosity, and Raman spectra of methanolic Mg (ClO4) 2, Mg (NO3) 2, and Mg (OAc) 2 solutions. J Chem Eng Data 54(2):436–443
Wahab A, Mahiuddin S, Hefter G, Kunz W, Minofar B, Jungwirth P (2005) Ultrasonic velocities, densities, viscosities, electrical conductivities, Raman spectra, and molecular dynamics simulations of aqueous solutions of Mg (OAc) 2 and Mg (NO3) 2: Hofmeister effects and ion pair formation. J Phys Chem B 109(50):24108–24120
Béléké AB, Mizuhata M, Kajinami A, Deki S (2003) Diffuse reflectance FT-IR spectroscopic study of interactions of α-Al 2 O 3/molten alkali nitrate coexisting systems. J Colloid Interface Sci 268:413–424
Su'ait MS, Ahmad A, Badri KH, Mohamed NS, Rahman MYA, Ricardo CA, Scardi P (2014) The potential of polyurethane bio-based solid polymer electrolyte for photoelectrochemical cell application. Int J Hydrog Energy 39:3005–3017
Reddy MJ, Chu PP (2002) Ion pair formation and its effect in PEO: Mg solid polymer electrolyte system. J Power Sources 109:340–346
Polu AR, Kumar R (2013) Preparation and characterization of pva based solid polymer electrolytes for electrochemical cell applications. Chin J Polym Sci 31:641–648
Sengwa RJ, Dhatarwal P, Choudhary S (2014) Role of preparation methods on the structural and dielectric properties of plasticized polymer blend electrolytes: correlation between ionic conductivity and dielectric parameters. Electrochim Acta 142:359–370
Boukamp BA (1986) A nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ionics 20(1):31–44
Boukamp BA (1986) A package for impedance/admittance data analysis. Solid State Ionics 18:136–140
Ramya CS, Selvasekarapandian S, Savitha T, Hirankumar G, Baskaran R, Bhuvaneswari MS, Angelo PC (2006) Conductivity and thermal behavior of proton conducting polymer electrolyte based on poly (N-vinyl pyrrolidone). Eur Polym J 42(10):2672–2677
Miyamoto T, Shibayama K (1973) Free-volume model for ionic conductivity in polymers. J Appl Phys 44(12):5372–5376
MacCallum JR, Vincent CA (eds.) (1989) Polymer electrolyte reviews vol. 2. Springer Science & Business Media, Berlin
Teeters D, Neuman RG, Tate BD (1996) The concentration behavior of lithium triflate at the surface of polymer electrolyte materials. Solid State Ionics 85(1–4):239–245
Evans J, Vincent CA, Bruce PG (1987) Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28(13):2324–2328
Kumar Y, Hashmi SA, Pandey GP (2011) Ionic liquid mediated magnesium ion conduction in poly (ethylene oxide) based polymer electrolyte. Electrochim Acta 56(11):3864–3873
Kumar GG, Munichandraiah N (2002) Poly (methylmethacrylate)—magnesium triflate gel polymer electrolyte for solid state magnesium battery application. Electrochim Acta 47(7):1013–1022
Pandey GP, Hashmi SA (2009) Experimental investigations of an ionic-liquid-based, magnesium ion conducting, polymer gel electrolyte. J Power Sources 187(2):627–634
TianKhoon L, Ataollahi N, Hassan NH, Ahmad A (2016) Studies of porous solid polymeric electrolytes based on poly (vinylidene fluoride) and poly (methyl methacrylate) grafted natural rubber for applications in electrochemical devices. J Solid State Electrochem 20(1):203–213
Pandey GP, Agrawal RC, Hashmi SA (2011) Performance studies on composite gel polymer electrolytes for rechargeable magnesium battery application. J Phys Chem Solids 72(12):1408–1413
Rosdi A, Zainol NH, Osman Z (2016) In: AIP Conference Proceedings, vol. 1711 no. 1, AIP Publishing, p 050003
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Mangalam R, Premalatha M, Monisha S (2017) Mg-ion conducting blend polymer electrolyte based on poly (vinyl alcohol)-poly (acrylonitrile) with magnesium perchlorate. Solid State Ionics 308:90–100
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Selvin PC, Mangalam R, Monisha S (2017) Preparation and characterization of blend polymer electrolyte film based on poly (vinyl alcohol)-poly (acrylonitrile)/MgCl2 for energy storage devices. Ionics. https://doi.org/10.1007/s11581-017-2273-9
Reddy CVS, Sharma AK, Rao VN (2003) Conductivity and discharge characteristics of polyblend (PVP+ PVA+ KIO 3) electrolyte. J Power Sources 114(2):338–345
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manjuladevi, R., Selvasekarapandian, S., Thamilselvan, M. et al. A study on blend polymer electrolyte based on poly(vinyl alcohol)-poly (acrylonitrile) with magnesium nitrate for magnesium battery. Ionics 24, 3493–3506 (2018). https://doi.org/10.1007/s11581-018-2500-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2500-z