Abstract
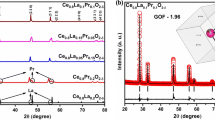
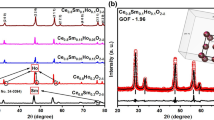
Nanocrystalline acceptor (Pr3+) and donor (Nb5+) doped cerium oxide are synthesized by sol-gel method via hydrolysis process and evaluated for the suitability of applying as an electrolyte for the intermediate temperature solid oxide fuel cells. Phase purity and crystallite size of the synthesized materials are ascertained by powder X-ray diffraction studies. Introduction of Pr3+ ions in the cerium lattice exhibited a lower crystallite size than the Nb5+, which exposes the probability to attain high oxide ion conductivity of Pr3+ doped cerium oxide. Fourier transform infrared and Raman spectra confirm the functional groups and formation of Pr3+ and Nb5+ ions in the cerium lattice. Absorbance spectra exhibit the charge-transfer transition from O2− (2p) to Ce4+ (4f) orbital in cerium oxide. Pr3+ and Nb5+ ions doped cerium lattice create the oxygen vacancies and favor the formation of Ce3+ from Ce4+. Valence band transition of Ce3+ ions from the 5d to 4f levels are examined by photoluminescence studies. The morphological features of Ce-Pr-O and Ce-Nb-O are investigated by scanning and transmission electron microscopy. Electrochemical impedance spectroscopy is used to analyze the conductivity properties of solid electrolytes. Ce-Pr-O shows the high oxide ion conductivity of 0.1 S/cm at 600 °C with an activation energy of 0.73 eV. Electrolytes with specific conductivities higher than 10−2 S/cm at intermediate temperatures (∼400–600 °C) are required for solid oxide fuel cells to operate with less maintenance. Hence, Ce-Pr-O can be a suitable electrolyte material for intermediate temperature solid oxide fuel cells.











Similar content being viewed by others
References
Venkatasubramanian A, Gopalan P, Prasanna TRS (2010) Synthesis and characterization of electrolytes based on BaO–CeO2–GdO1.5 system for intermediate temperature solid oxide fuel cells. Int J Hydrog Energy 35:4597–4605
Gnanam S, Rajendran V (2011) Synthesis of CeO2 or a–Mn2O3 nanoparticles via sol–gel process and their optical properties. J Sol-Gel Sci Technol 58:62–69
Mukherjee ST, Bedekar V, Patra A, Sastry PU, Tyagi AK (2008) Study of agglomeration behavior of combustion-synthesized nano-crystalline ceria using new fuels. J Alloys Compd 466:493–497
Goharshadi EK, Samiee S, Nancarrow P (2011) Fabrication of cerium oxide nanoparticles: characterization and optical properties. J Colloid Interface Sci 356:473–480
Tok AIY, Boey FYC, Dong Z, Sun XL (2002) Hydrothermal synthesis of CeO2 nano-particles. J Mater Process Technol 190:217–222
Kharton VV, Figueiredo FM, Navarro L, et al. (2001) Ceria-based materials for solid oxide fuel cells. J Mater Sci 36:1105–1117
Boudghene Stambouli A, Traversa E (2002) Solid oxide fuel cells (SOFCs): a review of an environmentally clean and efficient source of energy. Renew Sust Energ Rev 6:433–455
Mark Ormerod R (2003) Solid oxide fuel cells. Chem Soc Rev 32:17–28
Shao Z, Sossina M (2004) Haile a high-performance cathode for the next generation of solid-oxide fuel cells. Nature 431:170–173
Singhal SC (2000) Advances in solid oxide fuel cell technology. Solid State Ionics 135:305–313
Yamamoto O (2000) Solid oxide fuel cells: fundamental aspects and prospects. Electrochim Acta 45:2423–2435
Xia Y, Bai Y, Wu X, Zhou D, Liu X, Meng J (2011) The competitive ionic conductivities in functional composite electrolytes based on the series of M-NLCO (M = Ce0.8Sm0.2O2-δ, Ce0.8Gd0.2O2-δ, Ce0.8Y0.2O2-δ; NLCO =0.53Li2CO3 - 0.47Na2CO3). Int J Hydrog Energy 36:6840–6850
Shih S-J, Wu Y-Y, Borisenko KB (2011) Control of morphology and dopant distribution in yttrium-doped ceria nanoparticles. J Nanopart Res 13:1–8
Dikmen S, Aslanbay H, Dikmen E, Sahin O (2010) Hydrothermal preparation and electrochemical properties of Gd3+ and Bi3+, Sm3+, La3+, and Nd3+ codoped ceria-based electrolytes for intermediate temperature-solid oxide fuel cell. J Power Sources 195:2488–2495
Datta P, Majewski P, Aldinger F (2009) Study of gadolinia-doped ceria solid electrolyte surface by XPS. Mater Charact 60:138–143
Guan X, Zhou H, Liu Z, Wang Y, Zhang J (2008) High performance Gd3+ and Y3+ co-doped ceria-based electrolytes for intermediate temperature solid oxide fuel cells. Mater Res Bull 43:1046–1054
Ando M, Oikawa I, Noda Y, Ohki S, Tansho M, Shimizu T, Kiyono H, Maekawa H (2011) High field O-17 NMR study of defects in doped zirconia and ceria. Solid State Ionics 192:576–579
Fan Z, Chao C-C, Hossein-Babaei F, Prinz FB (2011) Improving solid oxide fuel cells with yttria-doped ceria interlayers by atomic layer deposition. J Mater Chem 21:10903–10906
Huijsman JPP, van Berkel FPF, Christie GM (1998) Intermediate temperature SOFC – a promise for the twenty-first century. J Power Sources 71:107–110
Maricle DL, Swarr TE, Karavolis S (1992) Enhanced ceria—a low-temperature SOFC electrolyte. Solid State Ionics 52:173–182
Song C (2002) Fuel processing for low-temperature and high-temperature fuel cells challenges, and opportunities for sustainable development in the twenty-first century. Catal Today 77:17–49
Zivkovic LS, Lair V, Lupan O, Cassir M, Ringuede A (2011) Samarium-doped ceria nanostructured thin films grown on FTO glass by electrodepostion. Acta Phys Pol A 120:298–302
Singh V, Babu S, Karakoti AS, Agarwal A, Seal S (2010) Effect of submicron grains on ionic conductivity of nanocrystalline doped ceria. J Nanosci Nanotechnol 10:1–9
Ramesh S, James Raju KC, Vishnuvardhan Reddy C (2011) Synthesis and characterization of co-doped ceria ceramics by sol-gel method. Trans Ind Ceram Soc 70:143–147
Ninic M, Boskovic S, Nenadovic M, Zec S, Voisavljevic K, Minic D, Matovic B (2007) Cerium oxide based nanometric powders: synthesis and characterization. Sci Sinter 39:301–308
Ou DR, Mori T, Ye F, Takahashi M, Zou J, Drennan J (2006) Microstructures and electrolytic properties of yttrium-doped ceria electrolytes: dopant concentration and grain size dependences. Acta Mater 54:3737–3746
Wang DY, Park DS, Griffith J, Nowick AS (1981) Oxygen-ion conductivity and defect interactions in yttria-doped ceria. Solid State Ionics 2:95–105
Huang W, Shuk P, Greenblattz M (2007) Hydrothermal synthesis and electrical characterization of (Ce0.83Sm0.17)1-x Ln x O2-δ (Ln = Pr, Tb) as potential electrolyte materials for solid oxide fuel cells. J Electrochem Soc 147:439–443
Venkatesh V, Vishnuvardhan Reddy C (2013) Effect of Y on the properties of Sm-doped ceria for IT-SOFC applications. J Mod Phys 4:1499–1503
Naik IK, Tien TY (1979) Electrical conduction in Nb2O5-doped cerium dioxide. J Electrochem Soc 126:562–566
Huang W, Shuk P, Greenblatt M (1997) Hydrothermal synthesis and properties of Ce1-xSmxO2-x/2 and Ce1-xCaxO2-x solid solutions. Chem Mater 9:2240–2245
Hongyun J, Wang N, Xu L, Hou S (2010) Synthesis and conductivity of cerium oxide nanoparticles. Mater Lett 64:1254–1256
Mori T, Drennan J (2006) Influence of microstructure on oxide ionic conductivity in doped CeO2 electrolytes. J Electroceram 17:749–757
Masui T, Fujiwara K, Machida K-i, Adachi G-y (1997) Characterization of cerium (IV) oxide ultrafine particles prepared using reversed micelles. Chem Mater 9:2197–2204
Laberty-Robert C, Long JW, Lucas EM, Pettigrew KA, Stroud RM, Doescher MS, Rolison DR (2006) Sol-gel-derived ceria nanoarchitectures: synthesis, characterization and electrical properties. Chem Mater 18:50–58
Alexander L, Klug HP (1950) Determination of crystallite size with the X-ray spectrometer. J Appl Phys 21:137–142
Patterson AL (1939) The Scherrer formula for X-ray particle size determination. Phys Rev 56:978–982
Jayaraman T, Raja SA, Priya A, Jagannathan M, Ashokkumar M (2015) Synthesis of a visible-light active V2O5-g-C3N4 heterojunction as an efficient photocatalytic and photoelectrochemical material. New J Chem 39:1367–1374
Amarsingh Bhabu K, Dhivya Saranya J, Rajasekaran TR (2013) Preparation and characterization of Ce0.8Y0.2O2 nanopowders using sol-gel method. Int J Mod Phys Conf Ser 22:533–544
de Larramendi IR, Ortiz-Vitoriano N, Acebedo B, de Aberasturi DJ, de Muro IG, Arango A, Rodrıguez-Castellon E, de Larramendi JIR, Rojo T (2011) Pr-doped ceria nanoparticles as intermediate temperature ionic conductors. Int J Hydrog Energy 36:10981–10990
Maso N, Beltran H, Munoz R, Julian B, Carda JB, Escribano P, Cordoncillo E (2003) Optimization of praseodymium-doped cerium pigment synthesis temperature. J Am Ceram Soc 86:425–430
Olegario RC, de Souza ECF, Borges JFM, da Cunha JBM, de Andrade AVC, Antunes SRM, Antunes AC (2013) Synthesis and characterization of Fe3+ doped cerium-praseodymium oxide pigments. Dyes Pigments 97:113–117
Ftikos C, Nauer M, Steele BCH (1993) Electrical conductivity and thermal expansion of ceria doped with Pr, Nb and Sn. J Eur Ceram Soc 12:267–270
Wang Y, Mori T, Li J-G, Ikegami T (2002) Low temperature synthesis of praseodymium doped ceria nanopowders. J Am Ceram Soc 85:3105–3107
da Fonseca RO, da Silva AAA, Signorelli MRM, Rabelo-Neto RC, Noronha FB, Simoesa RCC, Mattos LV (2014) Nickel / doped ceria solid oxide fuel cell anodes for dry reforming of methane. J Braz Chem Soc 25:2356–2363
Shrestha S, Yeung CMY, Nunnerley C, Tsang SC (2007) Comparison of morphology and electrical conductivity of various thin films containing nano-crystalline praseodymium oxide particles. Sens Actuators A 136:191–198
Ocana M (2002) Preparation and properties of uniform praseodymium doped ceria colloidal particles. Colloid Polym Sci 280:274–281
Liu YH, Zuo JC, Ren XF, Yong L (2014) Synthesis and character of cerium oxide (CeO2) nanoparticles by the precipitation method. Metalurgija 53:463–465
Chelliah M, Rayappan JBB, Krishnan UM (2012) Synthesis and characterization of cerium oxide nanoparticles by hydroxide mediated approach. J Appl Sci 12:1734–1737
Pragash R, Gijo J, Unnikrishnan NV, Sudarsanakumar C (2011) Energy transfer and thermal studies of Pr3+ doped cerium oxalate crystals. Bull Mater Sci 34:955–961
Li G, Li L, Jiang D (2015) Facile synthesis of highly active mesoporous PdCeOx solid solution for low-temperature CO oxidation. J Phys Chem C 119:12502–12507
Godinho MJ, Goncalves RF, Santos LPS, Varela JA, Longo E, Leite ER (2007) Room temperature co-precipitation of nanocrystalline CeO2 and Ce0.8Gd0.2O1.9−δ powder. Mater Lett 61:1904–1907
Hungria AB, Martinez-Arias A, Fernandez-Garcia M, Iglesias-Juez A, Guerrero-Ruiz A, Calvino JJ, Conesa JC, Soria J (2003) Structural, morphological and oxygen handling properties of nanosized cerium-terbium mixed oxides prepared by microemulsion. Chem Mater 15:4309–4316
Esther Jeyanthi C, Siddheswaran R, Pushpendra K, Singh J, Hui KN, Hui KS, Rajarajan K (2016) Synthesis, characterization and spectroscopic analysis of er-doped ceria (Ce0.9Er0.1O1.95) electrolyte for solid oxide fuel cells. Energy Environ Focus 3:196–201
Bondioli F, Corradi AB, Manfredin T (2000) Nonconventional synthesis of praseodymium-doped ceria by flux method. Chem Mater 12:324–330
Samiee S, Goharshadi EK, Nancarrow P (2012) Optical Properties of Ceria Nanoparticles. Proceedings of the 4th international conference on nanostructures, Kish Island, I.R. Iran 1222–1224
Shehata N, Meeran K, Hudait M, Jain N (2012) Control of oxygen vacancies and Ce3+ concentrations in doped ceria nanoparticles via the selection of lanthanide element. J Nanopart Res 14:1–10
Radhika S, Sreeram KJ, Nair BU (2014) Effective synthesis route for red-brown pigments based on Ce – Pr – Fe – O and their potential application for near infrared reflective surface coating. J Chem Sci 126:65–73
Kim JJ, Bishop SR, Thompson N, Kuru Y, Tuller HL (2012) Optically derived energy band gap states of Pr in ceria. Solid State Ionics 225:198–200
Cabral AC, Cavalcante LS, Deus RC, Longo E, Simoes AZ, Moura F (2014) Photoluminescence properties of praseodymium doped cerium oxide nanocrystals. Ceram Int 40:4445–4453
Wang Z, Quan Z, Lin J (2007) Remarkable changes in the optical properties of CeO2 nanocrystals induced by lanthanide ions ooping. Inorg Chem 46:5237–5242
Balakrishnan G, Raghavan CM, Ghosh C, Divakar R, Mohandas E, Song JI, Bae SI, Kim TG (2013) X-ray diffraction, Raman and photoluminescence studies of nanocrystalline cerium oxide thin films. Ceram Int 39:8327–8333
Amarsingh Bhabu K, Theerthagiri J, Madhavan J, Balu T, Muralidharan G, Rajasekaran TR (2016) Enhanced electrochemical behavior of ceria based zirconia electrolytes for intermediate temperature solid oxide fuel cells. J Mater Sci Mater Electron. doi:10.1007/s10854-016-5214-x (Article in Press)
Florea M, Avram D, Cojocaru B, Tiseanu I, Parvulescu V, Tiseanu C (2016) Defects induced tunable infrared emission of Er-CeO2 by heterovalent co-dopants. Phys Chem Chem Phys DOI. doi:10.1039/C6CP02754G (Article in Press)
Ravi Chandran P, Arjunan TV, Gokila Krishnan G, Tirumalaisamy S (2014) Investigation on Mg and Sc doped ceria for intermediate temperature solid oxide fuel cell. JMME-IJENS 14:98–103
Ramesh S, James Raju KC (2012) Preparation and characterization of Ce1-x(Gd0.5Pr0.5)xO2 electrolyte for IT-SOFCs. Int J Hydrog Energy 37:10311–10317
Acharya SA (2012) The effect of processing route on sinterability and electrical properties of nano-sized dysprosium-doped ceria. J Power Sources 198:105–111
Singh NK, Singh P, Kumar D, Parkash O (2012) Electrical conductivity of undoped, singly doped and co-doped ceria. Ionics 18:127–134
Omar S, Wachsman ED, Jones JL, Nino JC (2009) Crystal structure-ionic conductivity relationships in doped ceria systems. J Am Ceram Soc 92:2674–2681
Acknowledgments
One of the authors (K. Amarsingh Bhabu) is grateful to the University Grants Commission (UGC), Government of India, for the award of research fellowship under the UGC-SAP-Basic Science Research Program. Authors also thank Mr. M. Veera Gajendra Babu, Research Scholar, Department of Physics, Manonmaniam Sundaranar University, for his valid discussions regarding Rietveld Refinement Studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhabu, K.A., Theerthagiri, J., Madhavan, J. et al. Investigations on acceptor (Pr3+) and donor (Nb5+) doped cerium oxide for the suitability of solid oxide fuel cell electrolytes. Ionics 22, 2461–2470 (2016). https://doi.org/10.1007/s11581-016-1780-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1780-4