Abstract
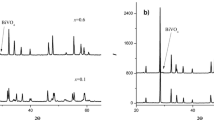
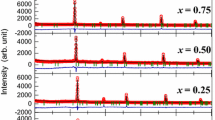
A series of complex oxides Bi13-xMexMo5O34±δ, Me = Mg, Ca, Sr, Ba were synthesized and studied. The solid solutions ranges and polymorphic modifications limits were determined. High temperature X-ray powder diffraction measurements showed the deviation of unit cell parameters dependence versus temperature from the linear function. Processes of forming of ceramic samples based on the synthesized powders were analyzed, and dense ceramic samples were obtained. Impedance spectroscopy displayed the changes of slope of Arrhenius plots which correspond with the HT-XPRD measurements. The presence of two different forms of monoclinic modification was supposed. The studied materials are promising ionic conductors, with the highest conductivity values about 6 × 10−3 S cm−1 at 973 K and 1.4 × 10−4 S cm−1 at 623 K.










Similar content being viewed by others
References
Boivin J-C (2001) Structural and electrochemical features of fast oxide ion conductors. Int J Inorg Mat 3:1261–1266. doi:10.1016/s1466-6049(01)00118-0
Grasselli RKJ (1986) Selective oxidation and ammoxidation of olefins by heterogeneous catalysis. Chem Educ 63:216–221. doi:10.1021/ed063p216
Keulks GW, Krenzke LD, Notermann TM (1979) Selective oxidation of propylene. Adv Catal 27:183–225. doi:10.1016/s0360-0564(08)60056-5
Libre JM, Barbaux Y, Grzybowska B, Gonflant P, Bonnelle JP (1983) Catalytic oxidation of propene: surface potential measurements and structural properties of α-Bi2Mo3O12, α-Bi2O3 and MoO3. Appl Catal 6:315–328. doi:10.1016/0166-9834(83)80104-3
Egashira M, Matsuo K, Kagawa S, Seiyama T (1979) Phase diagram of the system Bi2O3 - MoO3. J Catalysis 58:409–418. doi:10.1016/0021-9517(79)90279-3
Erman LY, Galperin EL, Kolchin IK (1964) The Bi2O3-MoO3-System. Russian J Inor Chem 9:1174–1176
Ono T, Utsumi K, Tsukamoto S, Tamaru H, Kataoka M, Noguchi F (2010) Roles of bulk γ(L)-Bi2MoO6 and surface β-Bi2Mo2O9 in the selective catalytic oxidation of C3H6. J Mol Catal A: Chemical 318:94–100. doi:10.1016/j.molcata.2009.11.012
Snyder TP, Hill CG (1991) Stability of bismuth molybdate catalysts at elevated temperatures in air and under reaction conditions. J Catalysis 132:536–555. doi:10.1016/0021-9517(91)90169-5
Crumpton TE, Francesconi MG, Greaves C (2003) The structural chemistry of Bi14 MO24 (M = Cr, Mo, W) phases: bismuth oxides containing discrete MO4 tetrahedra. J Solid State Chem 177:197–206. doi:10.1016/s0022-4596(03)00246-9
Sharma N, Macquart RB, Christensen M, Avdeev M, Chen YS, Ling CD (2009) Structure and crystal chemistry of fluorite-related Bi38Mo7O78 from single crystal X-ray diffraction and ab initio calculations. J Solid State Chem 182:1312–1318. doi:10.1016/j.jssc.2009.02.030
Valldor M, Esmaeilzadeh S, Pay-Gomez C, Grins J (2000) A new high-temperature cubic fluorite-type phase Mo0.16Bi0.84O1.74 with a rare three-dimensional incommensurate modulation. J Solid State Chem 152:573–576. doi:10.1006/jssc.2000.8742
Vila E, Landa-Canovas RA, Galy J, Iglesias JE, Castro A (2007) Bi2n+4MonO6(n+1) with n = 3, 4, 5, 6: A new series of low-temperature stable phases in the mBi2O3– MoO3 system (1.0 ≤ m ≤ 1.7): Structural relationships and conductor properties. J Solid State Chem 180:661–669. doi:10.1016/j.jssc.2006.10.036
Buttrey DJ, Vogt T, Yap GPA, Rheingold AL (1997) The structure of Bi26Mo10O69. Mater Res Bull 32:947–962. doi:10.1016/s0025-5408(97)00063-9
Vannier RN, Mairesse G, Abraham F, Nowogorski G (1996) Bi26Mo10Oδ solid solution type in the Bi2O3–MoO3–V2O5 ternary diagram. J Solid State Chem 122:394–406. doi:10.1006/jssc.1996.0133
Ling CD, Miiller W, Johnson MR, Richard D, Rols S, Madge J, Evans IR (2012) Local structure, dynamics, and the mechanisms of oxide ionic conduction in Bi26Mo10O69. Chem Mater 24:4607–4614. doi:10.1021/cm303202r
Ayame A, Uchida K, Iwataya M, Miyamoto M (2002) X-ray photoelectron spectroscopic study on α- and γ-bismuth molybdate surfaces exposed to hydrogen, propene and oxygen. Appl Catal A: General 227:7–17. doi:10.1016/s0926-860x(01)00918-8
Uchida K, Ayame (1996) A dynamic XPS measurements on bismuth molybdate surfaces. Surf Sci 357-358:170-175. doi: 0.1016/0039-6028(96)00083-0
Holmes L, Peng L, Heinma I, O’Dell LA, Smith ME, Vannier RN, Grey CP (2008) Variable-temperature 17O NMR study of oxygen motion in the anionic conductor Bi26Mo10O69. Chem Mater 20:3638–3648. doi:10.1021/cm800351c
Muktha B, Aarthi T, Madras G, Guru Row TN (2006) Substitution effect on the photocatalytic degradation by the series AxBi26-xMo10O68-0.5y (A = Ba, y = 0; A = Bi, La, y = 2): a kinetic study. J Phys Chem B 110:10280–10286. doi:10.1021/jp060945o
Galy J, Enjalbert R, Rozier P, Millet P (2003) Lone pair stereoactivity versus anionic conductivity. Columnar structures in the Bi2O3–MoO3 system. Solid State Sci 5:165–174. doi:10.1016/s1293-2558(02)00090-0
Enjalbert R, Hasselmann G, Galy J (1997) A New Mixed Oxide with (Bi12O14)n Columns: PbBi12Mo5O34. Acta Crystallgr C 53:269–272. doi:10.1107/s0108270196013698
Vannier RN, Danze S, Nowogrocki G, Huve M, Mairesse G (2000) A new class of mono-dimensional bismuth-based oxide anion conductors with a structure based on [Bi12O14]∞ columns. Solid State Ionics 136–137:51–59. doi:10.1016/S0167-2738(00)00351-9
Galy J, Salles P, Rozier P, Castro A (2006) Anionic conductors Ln2/3[Bi12O14](MoO4)5 with Ln = La, Nd, Gd, Ho, Yb. Synthesis–spark plasma sintering–structure–electric properties. Solid State Ionics 177:2897–2902. doi:10.1016/j.ssi.2006.07.059
Bastide B, Enjalbert R, Salles P, Galy J (2003) Ionic conductivity of the oxide family Bi[Bi12O14][(Mo, M)O4]5 with M = Li, Mg, Al, Si, Ge and V. Solid State Ionics 158:351–358. doi:10.1016/s0167-2738(02)00910-4
Mikhailovskaya ZA, Buyanova ES, Petrova SA, Morozova MV, Zhukovskiy VM, Zakharov RG, Tarakina NV, Berger IF (2013) Cobalt-doped Bi26Mo10O69: crystal structure and conductivity. J Solid State Chem 204:9–15. doi:10.1016/j.jssc.2013.05.006
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta cryst A 32:751–767. doi:10.1107/s0567739476001551
Irvine JTS, Sinclair DC, West AR (1990) Electroceramics: characterization by impedance spectroscopy. Adv Mater 2:132–138. doi:10.1002/adma.19900020304
Abrahams I, Krok F (2002) Defect chemistry of the BIMEVOXes. J Mater Chem 12:3351–3362. doi:10.1039/B203992N
Acknowledgments
This work was financially supported by the Russian Fund of Basic Research (project nos. 12-03-00464 and 14-03-92605)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mikhaylovskaya, Z.A., Buyanova, E.S., Morozova, M.V. et al. Bi13-xMexMo5O34±δ (Me = Mg, Ca, Sr, Ba) solid solutions: synthesis and properties. Ionics 21, 2259–2268 (2015). https://doi.org/10.1007/s11581-015-1421-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1421-3