Abstract
The cerebral collateral circulation is the main compensatory mechanism that maintains the ischemic penumbra viable, the tissue at risk for infarction that can be saved if blood flow is restored by reperfusion therapies. In clinical practice, the extent of collateral vessels recruited after vessel occlusion can be easily assessed with computed tomography angiography (CTA) using two different techniques: single-phase CTA (sCTA) and multi-phase CTA (mCTA). Both these methodologies have demonstrated a high prognostic predictive value for prognosis due to the strong association between the presence of good collaterals and favorable radiological and clinical outcomes in patients with acute ischemic stroke (AIS). However, mCTA seems to be superior to sCTA in the evaluation of collaterals and a promising tool for identifying AIS patients who can benefit from reperfusion therapies. In particular, it has recently been proposed the use of mCTA eligibility criteria has been recently proposed for the selection of AIS patients suitable for endovascular treatment instead of the current accepted criteria based on CT perfusion. In this review, we analyzed the characteristics, advantages and disadvantages of sCTA and mCTA to better understand their fields of application and the potential of mCTA in becoming the method of choice to assess collateral extent in AIS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The collateral circulation consists of the opening of alternative vascular channels distal to an occluded intracranial artery resulting in a massive vasodilatation that improves blood flow in hypoperfused brain regions. In acute ischemic stroke (AIS), collaterals represent the more important compensatory mechanism maintaining viable the ischemic penumbra, the reversibly damaged brain tissue at risk for infarction surrounding the irreversibly injured infarct core. The ischemic penumbra is the target of reperfusion therapies since it is potentially salvageable [1]. The prognostic value of collaterals in AIS patients is now generally accepted. Poor collaterals are predictors of unsuccessful recanalization and unfavorable clinical outcomes, while robust collaterals are associated with high recanalization rate, tissue reperfusion, early clinical improvement, small Final Infarct Volume (FIV), low risk of hemorrhagic transformation (HT) and favorable clinical outcomes [2, 3]. In addition, collaterals are associated with reduced ischemic core growth and allow the identification of patients showing rapid or delayed infarct expansion (namely fast or slow progressors) [4]. Therefore, collaterals could play a crucial role for the selection of patients candidates for intravenous thrombolysis (IVT) and endovascular treatment (EVT) in both early and late time windows [2]. Different imaging modalities are used for an appropriate evaluation of collateral extent that has become a major need in clinical practice [1, 2, 5, 6]. Digital subtraction angiography (DSA) is still considered as the gold standard for accurate identification of occlusion site and measurement of collaterals due to its high temporal and spatial resolution. In this setting, the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) scale [7, 8] and Careggi Collateral Score (CCS) [8,9,10] are some of the classifications used for collateral grading with DSA. However, DSA has many limitations since it is an invasive and time-consuming procedure, requires multiple acquisition to visualize both anterior and posterior collaterals and is at risk for thromboembolism. Information on collaterals can also be obtained with perfusion techniques, such as CTP and Magnetic Resonance Perfusion-Weighted Imaging (MR-PWI), using some multiparametric maps and, in particular, the Hypoperfusion Intensity Ratio (HIR) that is calculated on time-to-maximum of the tissue residue function (Tmax) maps as the quotient between the lesion volumes with Tmax > 10 s and > 6 s corresponding to the ratio between infarcted tissue and hypoperfused tissue, respectively. Nevertheless, although a strong correlation between HIR and collateral extent has been demonstrated, HIR does not allow a direct visualization of collaterals. Thus, based on these considerations, collateral assessment is currently performed with computed tomography angiography (CTA) that is a widely available, non-invasive, safe and therefore feasible method [2, 5, 6]. Single-phase CTA (sCTA) is able to identify the point of occlusion and collateral filling, but multi-phase CT angiography (mCTA) was recently proposed, consisting of a three-time points acquisition which provides a more detailed evaluation of collaterals and presents several advantages over sCTA. In fact, mCTA demonstrated a better correlation with FIV and functional outcome, and higher interrater reliability compared to sCTA. Moreover, several studies showed that mCTA is a useful tool for patient selection undergoing EVT, especially in the extended time window where the collateral assessment with mCTA could replace the current approach based on CT perfusion (CTP) parameters [6].
Functional anatomy of collateral circulation
The cerebral collateral circulation is a vascular system that regulates cerebral blood flow (CBF) in case of vessel occlusion and consists of a network of anastomoses belonging to both the arterial and venous circulation [1, 2, 11,12,13]. On the arterial side, three principal routes exist: (1) collaterals between the intracranial and the extracranial arteries provided by ophthalmic and superficial temporal arteries; (2) collaterals between the intracranial arteries based on the circle of Willis, a ring-shaped circuit of vessels in which the anterior communicating artery promotes interhemispheric blood flow and the posterior communicating arteries connecting the anterior and posterior circulation; (3) collaterals between the intracranial arteries provided by pial or leptomeningeal branches which generate a communication among the three large vessels of each hemisphere (anterior, middle and posterior cerebral arteries) supplying the cortical surface. At intracranial level, collaterals represented by the components of circle of Willis are indicated as primary, whereas pial (leptomeningeal) collaterals are considered as secondary because are usually recruited when primary collaterals fail. Of note, a variable anatomic configuration is typical of the circle of Willis since the anterior portion results to be complete in only 68%, the posterior portion in 47% and the entire circle in only 36% of individuals. In this regard, it has recently been suggested that the different structures of the circle of Wills could be implicated in defining fast progressors and could correlate with poor collateral score [14]. A high anatomic variability is also characteristic of venous collateral circulation collaterals that increases CBF drainage in case of occlusion of prominent pathways or venous hypertension and allows the exit of blood from the brain through multiple routes. Functionally, collateral activation is modulated by sympathetic system via intrinsic and extrinsic innervations which act on parenchymal vessels and vascular branches on the brain surface, respectively [12]. However, the two key events leading the opening of collaterals are the recruitment and the remodeling [1, 2, 11, 12]. The collateral recruitment can occur early, immediately or in minutes, or can be delayed, as typically occurs in chronic obstructive disease but sometimes appears within a few hours also after acute vessel occlusion providing a potential explanation for spontaneous acute tissue reperfusion. This recruitment is due to the drop of perfusion pressure in vessels downstream the occlusion with formation of a pressure gradient favoring the diversion of blood flow into collaterals and the shear stress that induces vasodilatation. Nevertheless, collateral recruitment is impaired by several conditions including advanced age, chronic hypertension, cerebral small vessel disease [1]. A greater lumen expansion of collaterals associated with increase in tortuosity, vessel length and wall thickness represents the collateral remodeling that develops in days or weeks, mainly during conditions characterized by a chronic decreased in CBF such as stenosis or occlusion of the internal carotid artery.
CTA acquisition and collateral grading
sCTA technique
sCTA images are obtained on a standard CT scanner through a volumetric acquisition starting approximately 5–10 s after automatic injection of 50–70 mL of a contrast bolus at the rate of 4–5 mL/s into an antecubital vein. sCTA source images are then reformatted with maximum intensity projection (MIP), a 3D reconstruction algorithm that provides images with a good anatomic detail of cerebral vessels and allows an accurate visualization of collaterals [5, 15]. Usually, sCTA covers from the aortic arch to vertex for the identification of occlusion site at the level of cervical and intracranial vessels (Fig. 1). Several grading scales have been proposed, but four collateral scores based on visual inspection of the degree of collateral filling are the most utilized grading systems in clinical practice (Table 1). The collateral grading system introduced by Miteff and coworkers [16] is a qualitative 3-point scale assigning 3 different grades of retrograde filling to the distal branches of middle cerebral artery (MCA) in the occluded territory that result in poor (grade 1), intermediate (grade 2) and good (grade 3) collaterals (Fig. 2, Panel A). The collateral score suggested by Tan and colleagues [17] is a qualitative a 4-point scale that classifies collateral supply filling of occluded middle cerebral artery territory in 4 different grades corresponding to poor (scores 0–1) and good (scores 2–3) collaterals (Fig. 2, Panel B). The collateral scoring system published by Mass and collaborators [18] is a qualitative 5-point scale that compares the opacification of sylvian and leptomeningeal vessels between occluded territory and contralateral normal side obtaining 5 different grades that represent poor (grade 1), intermediate (grade 2) and good (grades 3–5) collaterals (Fig. 2, Panel C). The collateral score described by Menon et al. [19] is a semiquantitative 20-point score that divides the territories supplied by anterior cerebral artery (ACA) and MCA in 9 regions attributing 0, 2 or 4 points to sylvian scissure and 0, 1 or 2 points to remaining areas after comparison of opacification of pial and lenticulostriate vessels between the occluded territory and contralateral normal side. Collaterals are judged as poor, intermediate and good with a scoring of 0–10 points, 11–16 points and 17–20 points, respectively (Fig. 2, Panel D). In all these collateral scores, except Tan grading, intermediate and good collaterals are considered as good. However, there is currently no consensus on the standard methodology to use for evaluating collateral extent since in prior publications, Menon scale performed better than Miteff score in predicting core and penumbra volumes likely because of its greater ability to estimate the delay rather than the backflow in the affected territory [20].
Single-phase CT angiography covers from the aortic arch to vertex and maximum intensity projection reconstructions for the visualization of cervical and intracranial vessels. CTA of the cervical and intracranial vessels was performed as follows: 0.7 mL/kg contrast (maximum 90 mL), 5- to 10-s delay from injection to scanning, 120 kV, 270 mA, 1 s/rotation, 1.25-mm-thick slices, and table speed 3.75 mm/rotation. The axial images were reconstructed at 1-mm overlapping sections, and multiplanar reconstructions for axial, coronal, and sagittal images of the circle of Willis were performed with 3 mm thickness at 1-mm intervals. Thick-section axial maximum intensity projections at 24 mm thickness and 4-mm intervals were also reconstructed
Single-phase CT angiography collateral scores. Green arrows indicate occlusion site or occluded hemisphere. M1, anterior MCA at ganglionic level; M2, lateral MCA at ganglionic level; M3, posterior MCA at ganglionic level; M4, anterior MCA at supraganglionic level; M5, lateral MCA at supraganglionic level; M6, posterior MCA at supraganglionic level; ACA, anterior cerebral artery; BG, basal ganglia
mCTA technique
mCTA is a three-phase acquisition offering time-resolved cerebral angiograms of brain vessels. The first phase replicates the only one phase of sCTA covering from aortic arch to vertex, whereas the following two phases are acquired from the skull base to vertex after table repositioning to the skull base for the visualization of intracranial vessels with a delay 8 s each (Fig. 3). There are no differences in the other characteristics of acquisition compared to sCTA. Therefore, the first phase corresponds to arterial phase, the second phase to equilibrium/venous or peak venous or phase and the third one to late venous phase [21]. mCTA can also be reconstructed from CTP peak of arterial phase obtaining three simulated mCTA phases [22]. In this case, however, the first phase includes only intracranial and not also cervical vessels as in the original mCTA acquisition. Using mCTA, collaterals are assessed with the grading system proposed by Menon and colleagues that is a qualitative 6-point scale (Table 2) in which collateral supply is categorized based on extent and prominence of vascular enhancement after comparison between the occluded territory and contralateral normal side and is identified as poor (grades 0–1), intermediate (grades 2–3) and good (grades 4–5) [21]. In contrast with sCTA classifications, mCTA Menon score defines intermediate profile as poor and not as good collaterals. Of note, while this classification was fully applied in several studies evaluating collaterals with mCTA [23,24,25,26,27,28,29,30], in others collateral filling was measured with sCTA Menon score [17, 20, 29] or sCTA Tan grading [17, 31,32,33] and intermediate pattern was included in good collaterals. Furthermore, grade 2 instead of grade 4, as stated in the original publication of Menon et al., was found to be the optimal threshold for identifying a good functional independence [27]. Thus, the more accurate score for calculating mCTA collateral extent has to be definitely validated.
Multiphase CT angiography covering from the carotid bifurcation to vertex (first phase) and from the skull base to vertex (second and third phases) with maximum intensity projection (MIP) reconstructions for the visualization of cervical and intracranial vessels (first phase) and intracranial vessels only (second and third phases). Green arrow indicates occlusion site. CTA of the cervical and intracranial vessels was performed as follows: 0.7 mL/kg contrast (maximum 90 mL), 5- to 10-s delay from injection to scanning, 120 kV, 270 mA, 1 s/rotation, 0.625-mm-thick slices, and table speed 3.75 mm/rotation. The second phase was acquired after a delay of 8 s that allows for table repositioning to the skull base. Scanning duration for each additional phase was 3.4 s. Thus, the three phases were each 12 s apart. The axial images were reconstructed at 1-mm overlapping sections, and multiplanar reconstructions for axial, coronal, and sagittal images of the circle of Willis were performed with 3 mm thickness at 1-mm intervals. Thick-section axial maximum intensity projections at 24 mm thickness and 4-mm intervals were also reconstructed
Automated collateral detection
Several efforts have recently been made to obtain an automated assessment of collateral extent and provide a reliable quantitative collateral scoring, mainly based on artificial intelligence programs. For sCTA, a good agreement was found between visual grading scale proposed by Tan and an automated collateral score performed using in-house [34, 35] or commercial software algorithms, such as Brainomix [36], StrokeViewer [37] and Canon [38]. In addition, a study analyzing patients from MR CLEAN database with in-house software showed that quantitative score was an independent predictor of functional outcome and FIV [34]. For mCTA, the most used automated method is provided by GE Healthcare FastStroke software generating time-variant color-coded maps which correlated well with conventional mCTA collateral score for the evaluation of collateral flow [38, 39], improved the interpretation of collateral status [40] and enhanced the predictive value of collaterals for good outcome [41]. Another in-house software with a good performance for the measurement of collateral supply was more recently described using CTP-derived mCTA images [42]. However, a validation on a larger population of patients is needed before the introduction of these automated software in clinical practice.
CTA collateral assessment in AIS
A large number of previous studies demonstrated the high predictive value of good collaterals assessed by sCTA for favorable clinical outcome, small FIV and low rates of HT in AIS patients treated with IVT and EVT [43,44,45]. These findings were confirmed and expanded in a series of recent publications using both sCTA and mCTA techniques, mainly in patients that underwent EVT.
sCTA collaterals
A clear association between intermediate and good collaterals and favorable clinical outcome was found in a post hoc analysis of Interventional Management of Stroke (IMS) III trial evaluating sCTA collaterals with Maas and Tan scores in AIS patients treated with IVT within 3 h or combined IVT and EVT within 7 h from symptom onset [46]. The same correlation between good collaterals and functional independence was observed in two post hoc analyses of MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands) [47] and DAWN (Triage of Wake-up and Late Presenting Strokes Undergoing Neurointervention With Trevo) [48] trials which measured sCTA collaterals using Tan score in patients receiving EVT at 6 h and at 6–24 h after stroke, respectively. However, benefit from EVT was also seen in patients with poor collaterals rated with Tan scale in an analysis of MR CLEAN Registry [49] and in HERMES (Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials) meta-analysis including patients who underwent EVT with or without IVT from 4.5 to 12 h after symptom onset [50]. Conversely, in a post hoc analysis of DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) no association was reported between sCTA collaterals graded with Maas and Tan scores and clinical outcome in patients treated with EVT at 6–16 h from onset [51]. Next, some investigations showed that good collaterals classified on sCTA with Tan scale predicted successful recanalization after IVT within 4.5 h [52] and EVT up to 24 h [48, 53] after onset, whereas the beneficial effect on clinical outcome of an earlier time to recanalization was independent of collaterals evaluated with Tan score in an analysis of MR CLEAN Registry [54] and detected only in patients with poor collaterals assessed by Miteff scale in a study based on a Korean Registry including patients treated with EVT within 6 h after onset [55]. Nevertheless, in a further analysis of the MR CLEAN Registry successful recanalization after EVT was not affected by sCTA collateral status graded by Tan score [56]. In addition, some studies revealed the predictive value of poor sCTA collaterals scored with Tan and Miteff scales for larger FIV as defined on non-contrast CT (NCCT) or Diffusion-Weighted Imaging (MR-DWI) [48, 57, 58] and increased HT rates [59, 60] in patients treated with EVT up to 24 h from onset. A robust association of good sCTA collaterals with reduced core growth and slow infarct progression was then identified in several studies including untreated patients and patients undergoing EVT within 24 h after onset where Tan [51, 61,62,63,64], Maas [65], Menon [66] and Miteff [67] were used as grading scores. In this setting, it is interesting to note that while poor collaterals were associated with penumbral salvage in a population of not treated patients [61, 67], an analysis of Acute Stroke Registry and Analysis of Lausanne (ASTRAL) did not document any correlation between penumbra volume determined by CTP and sCTA collaterals assessed with Tan score in untreated and IVT and/or EVT-treated patients admitted within 24 h from onset, suggesting that the relationship between collaterals and tissue at risk for infarction remains to be completely clarified [68]. Overall, these data indicate that sCTA collateral assessment is an important tool for predicting radiological and clinical outcomes in AIS patients receiving IVT and/or EVT in early and late time windows. On the other hand, sCTA collaterals were also associated with clot characteristics as patients with good collateral score had longer thrombi [69] and elevated thrombus permeability [70], reflecting the presence of residual blood flow through the clot. Finally, a lower edema progression and a greater benefit from EVT in patients with large core have been reported in patients with good sCTA collateral filling [71, 72].
mCTA collaterals
In the seminal work of Menon and associates the predictive value of mCTA collateral score for functional outcome was modest in AIS patients untreated or treated with IVT and EVT with or without IVT within 12 h of symptom onset [21]. This not excellent association of mCTA collateral assessment with prognosis was confirmed in two recent studies comparing the precision of different grading system applied to mCTA in identifying the degree of functional independence in patients receiving EVT in the same time window. In fact, no significant correlation with clinical outcome was seen for mCTA Menon and sCTA Miteff, Tan and Maas classifications in the first investigation [73], as well as for sCTA Menon and Tan scores in the HERMES analysis [74]. However, the evaluation of collateral filling with mCTA was successfully used as selection tool for AIS patients’ candidates for EVT within 12 h from stroke in the Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) trial [31] where patients with good collaterals undergoing EVT achieved more frequently a favorable outcome than controls treated with standard medical therapy. In agreement with these findings, data coming from subsequent studies documented the ability of mCTA scoring system in the prediction of functional outcome. A significant association between good mCTA collaterals and functional independency at 3 months was observed in patients treated within 4.5 after onset with EVT with or without IVT [25] and treated with standard medical therapy, IVT and/or EVT [24]. Next, recent publications showed that patients untreated or treated with EVT and/or IVT within 5 h [75], between 5 and 15 h [22] and within 24 h after ictus [28] who had a good mCTA collateral status at presentation achieved a favorable outcome. In addition, good mCTA collaterals were correlated with small FIV as delineated on NCCT or MR-DWI in patients receiving standard medical therapy, IVT and/or EVT within 4.5 [25], 12 [76, 77] and 24 h of symptom onset [28]. Another association was found between good mCTA collaterals and small admission infarct core as estimated on NCCT Alberta Stroke Program Early Computed Tomography Score (ASPECTS) methodology or MR-DWI in patients untreated or treated with IVT and/or EVT within 8 [26] and 14 h after onset [30]. Finally, good collaterals on mCTA were also linked with slow infarct growth rate in patients treated with IVT and/or EVT within 6 h from symptom onset and achieving successful recanalization [33]. In addition, poor mCTA collaterals were independent markers of malignant infarction defined as development of a large space-occupying brain edema involving at least 2/3 of the MCA territory with ventricles’ compression or midline shift in patients not treated or treated with IVT and/or EVT within 4.5 h from onset [23].
Superiority of mCTA over sCTA
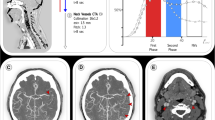
The main limitation of sCTA is the lack of temporal resolution since the acquisition based on a single time point can lead to an overestimation of collaterals when scans are obtained too late in the venous phase, due to a slow blood flow as a consequence of reduced cardiac output or cervical carotid artery stenosis, or an underestimation of collateral supply when scans are acquired too early in the arterial phase [2, 6, 15]. For the same reason, sCTA is unable to correctly visualize delayed collateral filling during the venous phase with the possibility of considering patients having good collaterals as patients with poor collaterals [6]. These misleading results can be overcome by mCTA that provides three time-resolved images allowing to explore collateral circulation not only in the arterial phase, but also in peak and late venous phases [6, 21]. As a consequence, mCTA ensures a more accurate collateral assessment than sCTA, avoiding misclassification due to the appearance of delayed filling of collaterals in venous phases (Fig. 4). In addition, mCTA shows other advantages over sCTA such as higher interrater reliability and, more important, a superior accuracy in predicting functional outcome at 3 months as demonstrated in the original publication by Menon and associates [21]. Other studies repeatedly confirmed that mCTA is superior to sCTA as prognostic predictor in patients admitted at 4.5–15 h after symptom onset and treated with standard medical therapy and IVT and/or EVT [22, 24, 75]. In this regard, the stronger demonstration that mCTA improves outcome prediction compared to sCTA has been reported by Wang and collaborators [28] where mCTA resulted independently associated with functional outcome in patients untreated and treated with IVT and/or EVT within 24 h after onset, whereas sCTA did not. Intriguingly, mCTA outperforms sCTA also in the analysis of venous outflow at the level of cortical veins representing an indirect indicator of collateral extent and tissue perfusion. In fact, venous drainage reflects the ability of cortical venous system in containing the increased blood volume due to the opening of collaterals and then the effective blood flow traffic through cerebral microcirculation in the ischemic territory [1, 11, 78,79,80]. sCTA usually determines cortical venous filling according to the Cortical Vein Opacification Score (COVES) proposed by Jansen et al. in a post hoc analysis of MR CLEAN trial that is a qualitative 6-point scale in which the opacification of the superficial middle cerebral vein, the vein of Labbé and the sphenoparietal sinus is classified as complete absence (grade 0), moderate (grade 1) and full (grade 2) after comparison between occluded and unaffected territories. Grades 0–2 indicate poor venous outflow, whereas Grades 3–6 are considered good venous outflow [81]. Several publications reported a strong association of favorable venous outflow with good functional outcome, good collaterals, successful recanalization, lower risk for HT and lower edema progression in AIS patients receiving EVT with or without IVT between 6 and 16 h after symptom onset [80,81,82,83,84,85,86,87]. However, Singh and associates recently analyzed patients treated with IVT and/or EVT within 12 h from onset enrolled in Rapid Assessment of Collaterals Using Multi-Phase CTA in the Triage of Patients with Acute Ischemic Stroke for IV or IA Therapy (PRove-IT) multicenter cohort study [88]. They demonstrated that COVES also including the opacification of the vein of Trolard was more robust as outcome determinant than sCTA COVES. This novel classification, described as total venous score (TVS), is a qualitative 24-point scale in which the filling of the superficial middle cerebral vein, the vein of Labbé, the sphenoparietal sinus and the vein of Trolard is graded as no (grade 0), partial (grade 1) and full (grade 2) opacification in all three phases. The final score is generated by adding the opacification scores calculated in each mCTA phase. In particular, this study documented the relevance of venous opacification during mCTA second and third phases in the actual evaluation of venous filling, suggesting that venous outflow is a time-dependent phenomenon (Fig. 5). Based on these findings, although not without drawbacks as collateral filling could be reduced by flow-limiting proximal stenosis and poor cardiac function [6, 21], mCTA is currently considered the method of choice for collateral assessment in early (0–6 h) and late (6–24 h) time windows for EVT [6].
Multiphase CT angiography evaluates collaterals better than single-phase CT angiography. In the first arterial phase corresponding to single-phase CT angiography acquisition collaterals are erroneously judged as poor. On the contrary, the second peak venous phase reveals that collaterals are good. Green arrow shows occlusion site. Yellow arrows indicate collateral extent in the occluded hemisphere
Multi-phase CTA collateral-based selection for EVT
DEFUSE 3 [89] and DAWN [90] randomized controlled trials (RCTs) have recently demonstrated the utility of CTP in the identification of AIS patients suitable for EVT in late time window (6–24 h) and patients selected for EVT because of a favorable CTP profile achieved a good outcome more frequently than controls treated with standard care. In both trials, patients were treated if they satisfied specific optimal CTP-derived parameters, collectively named target mismatch, automatically calculated with a dedicated software (RAPID; Rapid Processing of Perfusion and Diffusion; iSchemaView, Menlo Park, CA) using prespecified CTP thresholds. More precisely, absolute time to the peak of the residual function (Tmax) values more than 6 s (Tmax > 6 s) was assumed to indicate critically hypoperfused tissue and relative cerebral blood flow (CBF) values less than 30% of normally perfused tissue (rCBF < 30%) were considered to delineate infarct core. However, despite the successful results of these RCTs, futile recanalization rates were of about 50% [91], suggesting that selection strategy should be improved. ESCAPE trial [31] showed that an mCTA-based selection for EVT could be a promising alternative. Therefore, some research groups have started to explore whether CTA was non-inferior to CTP in the identification of AIS patients who can benefit from EVT. mCTA was initially compared to CTP DEFUSE 3 and DAWN eligibility criteria for the selection of patients’ candidates for reperfusion therapies in two studies evaluating patients treated with EVT or standard medical therapy at 6–12 h after symptom onset from Prove-IT dataset [92] and at 6–24 h after stroke in a Korean single center [93]. In both studies, good collaterals and a good CTP profile were equivalent in predicting favorable outcome. A subsequent study reported that mCTA collaterals were not inferior to perfusion in determining outcome in patients treated with IVT and/or EVT within 24 h of onset [28]. Other two investigations substantially confirmed these findings. Functional outcome was comparable in patients receiving EVT selected with NCCT ASPECTS and mCTA at 6–12 h after onset in ESCAPE Na1 trial [94] and with CTP at 6–16 h and at 6–24 h from onset in DEFUSE 3 [89] and DAWN [90] trial, respectively. More recently, in a Selection Of Late-window Stroke for Thrombectomy by Imaging Collateral Extent (SOLSTICE) Consortium pooled analysis including patients overlapping DEFUSE 3 and DAWN trials as baseline characteristics treated with EVT, the selection with collateral and perfusion imaging showed a similar predictive value for clinical outcome [95]. However, the latest study of Tan et al. did not replicate these results and demonstrated that CTP selection criteria were superior than mCTA eligibility criteria in predicting outcome [96]. Therefore, the possibility that mCTA collateral guided can replace CTP-guided criteria in the selection of patients suitable for EVT in the late time window (6–24 h) still remains matter of debate. In this regard, the relationships between mCTA collateral-based and CTP-based selection criteria were well summarized by Ospel and colleagues which collected data from patients untreated and treated with IVT and/or EVT within 12 h of onset enrolled in Prove-IT study [97]. In this publication, infarct core was defined using three threshold values (rCBF < 30%, CBF < 7 mL/100 g/min, 10 and CBV < 2 mL/100 g) obtaining different results for CTP-guided selection according to the different CTP thresholds utilized. Patients considered eligible for EVT with combined CTP (small core + large penumbra) and mCTA (good collaterals) achieved favorable outcome in 62–87% of cases. In addition, mCTA eligibility criteria selected more patients (91%) than CTP eligibility criteria (7%-36%), but with lower good outcome rates (53–57%). Surprisingly, 51–62% of patients who were not eligible by either mCTA or CTP achieved a good outcome. Therefore, these findings suggest that, although the selection criteria are currently limited, the integration between mCTA collateral-based and CTP-based selection criteria could represent the best paradigm.
Future perspectives
In line with the observations emerged from the study of Ospel et al. [97], the recently proposed opportunity to generate CTP maps (delay maps) from mCTA could allow to combine mCTA and CTP data for EVT patient selection with the advantage of reducing acquisition time and radiation dose [98, 99]. In the same way, it is now well-accepted that the fate of ischemic tissue depends not only on the amount of blood delivered by collaterals, but also on the blood volume flowing across the microcirculation and drained by venous system. Therefore, the simultaneous assessment of collateral extent by CTA with tissue-level collaterals by HIR and venous outflow by CTA, the so-called cerebral collateral cascade [100, 101], may be a further option for improving our ability to identify AIS patients who actually benefit from reperfusion therapies.
References
Patel SD, Liebeskind D (2023) Collaterals and elusive ischemic penumbra. Transl Stroke Res 14:3–12
Uniken Venema SM, Dankbaar JW, van der Lugt A, Dippel DWJ, van der Worp HB (2022) Cerebral collateral circulation in the era of reperfusion therapies for acute ischemic stroke. Stroke 53:3222–32343
Lee JS, Bang OY (2023) Collateral status and outcomes after thrombectomy. Transl Stroke Res 14:22–374
Seifert K, Heit JJ (2023) Collateral blood flow and ischemic core growth. Transl Stroke Res 14:13–21
Ginsberg M (2018) The cerebral collateral circulation: relevance to pathophysiology and treatment of stroke. Neuropharmacology 15(5):280–292
Dundamadappa S, Iyer K, Agrawal A, Choi DJ (2021) Multiphase CT angiography: a useful technique in acute stroke imaging-collaterals and beyond. AJNR Am J Neuroradiol 42:221–227
Liebeskind D (2003) Collateral circulation. Stroke 34:2279–2284
Liebeskind DS, Tomsick TA, Foster LD, Yeatts SD, Carrozzella J, Demchuk AM, Jovin TG, Khatri P, von Kummer R, Sugg RM, Zaidat OO, Hussain SI, Goyal M, Menon BK, Al Ali F, Yan B, Palesch YY, Broderick JP, IMS III Investigators (2014) Collaterals at angiography and outcomes in the interventional management of stroke (IMS) III trial. Stroke 45:759–764
Mangiafico S, Consoli A, Renieri L, Rosi A, De Renzis A, Vignoli C, Capaccioli L (2013) Semi-quantitative and qualitative evaluation of pial leptomeningeal collateral circulation in acute ischemic stroke of the anterior circulation: the Careggi Collateral Score. Ital J Anat Embryol 118:277–287
Mangiafico S, Saia V, Nencini P, Romani I, Palumbo V, Pracucci G, Consoli A, Rosi A, Renieri L, Nappini S, Limbucci N, Inzitari D, Gensini GF (2014) Effect of the interaction between recanalization and collateral circulation on functional outcome in acute ischaemic stroke. Interv Neuroradiol 20:704–714
Consoli A, Andersson T, Holmberg A, Verdant L, Saletti A, Vallone S, Zini A, Cerase A, Romano D, Bracco S, Lorenzano S, Fainardi E, Mangiafico S (2016) CT perfusion and angiographic assessment of pial collateral reperfusion in acute ischemic stroke: the CAPRI study. J Neurointerv Surg 8:1211–1216
Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS (2011) Collateral blood vessels in acute ischemic stroke: a potential therapeutic target. Lancet Neurol 10:909–921
Brozici M, Van der Zwan A, Hillen B (2003) Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke 34:2750–2762
Ospel JM, Hill MD, Kappelhof M, Demchuk AM, Menon BK, Mayank A, Dowlatshahi D, Frei D, Rempel JL, Baxter B, Goyal M (2021) Which acute ischemic stroke patients are fast progressors? results from the ESCAPE trial control arm. Stroke 52:1847–1850
Raymond SB, Schaefer PW (2017) Imaging brain collaterals: quantification, scoring, and potential significance. Top Magn Reson Imaging 26:67–75
Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW (2009) The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain 132:2231–2238
Tan IYL, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, Martin M, Symons SP, Fox AJ, Aviv RI (2009) CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol 30:525–531
Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, Harris GJ, Halpern E, Kemmling A, Koroshetz WJ, Furie KL (2009) Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke 40:3001–3005
Menon BK, Smith EE, Modi J, Patel SK, Bhatia R, Watson TW, Hill MD, Demchuk AM, Goyal M (2011) Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. AJNR Am J Neuroradiol 32:1640–1645
Seker F, Potreck A, Möhlenbruch M, Bendszus M, Pham M (2016) Comparison of four different collateral scores in acute ischemic stroke by CT angiography. J Neurointerv Surg 8:1116–1118
Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, Goyal M (2015) Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology 275:510–520
Lu SS, Zhang X, Xu XQ, Cao YZ, Zhao LB, Liu QH, Wu FY, Liu S, Shi HB (2019) Comparison of CT angiography collaterals for predicting target perfusion profile and clinical outcome in patients with acute ischemic stroke. Eur Radiol 29:4922–4929
Flores A, Rubiera M, Ribó M, Pagola J, Rodriguez-Luna D, Muchada M, Boned S, Seró L, Sanjuan E, Meler P, Carcámo D, Santamarina E, Tomassello A, Lemus M, Coscojuela P, Molina CA (2015) Poor collateral circulation assessed by multiphase computed tomographic angiography predicts malignant middle cerebral artery evolution after reperfusion therapies. Stroke 46:3149–3153
García-Tornel A, Carvalho V, Boned S, Flores A, Rodríguez-Luna D, Pagola J, Muchada M, Sanjuan E, Coscojuela P, Juega J, Rodriguez-Villatoro N, Menon B, Goyal M, Ribó M, Tomasello A, Molina CA, Rubiera M (2016) Improving the evaluation of collateral circulation by multiphase computed tomography angiography in acute stroke patients treated with endovascular reperfusion therapies. Interv Neurol 5:209–217
Uransilp N, Dharmasaroja PA, Watcharakorn A, Muengtaweepongsa S (2019) Implementation of multiphase computed tomography angiography in management of patients with acute ischemic stroke in clinical practice. J Clin Neurosci 62:100–104
Lee SJ, Jung WS, Choi MH, Hong JM, Lee JS, Choi JW (2019) Optimal multiphase computed tomographic angiography-based infarct core estimations for acute ischemic stroke. Sci Rep 9:15243
Woo HG, Jung C, Sunwoo L, Bae YJ, Choi BS, Kim JH, Kim BJ, Han MK, Bae HJ, Jung S, Cha SH (2019) Dichotomizing level of pial collaterals on multiphase CT angiography for endovascular treatment in acute ischemic stroke: should it be refined for 6-hour time window? Neurointervention 14:99–106
Wang Z, Xie J, Tang TY, Zeng CH, Zhang Y, Zhao Z, Zhao DL, Geng LY, Deng G, Zhang ZJ, Ju SH, Teng GJ (2020) Collateral status at single-phase and multiphase CT angiography versus CT perfusion for outcome prediction in anterior circulation acute ischemic stroke. Radiology 296:393–400
Sarraj A, Hassan AE, Grotta J, Blackburn S, Day A, Abraham M, Sitton C, Dannenbaum M, Cai C, Pujara D, Hicks W, Vora N, Budzik R, Shaker F, Arora A, Riascos RF, Kamal H, Martin-Schild S, Lansberg M, Gupta R, Albers GW, SELECT Investigators (2021) Early infarct growth rate correlation with endovascular thrombectomy clinical outcomes: analysis from the SELECT study. Stroke 52:57–69
Laflamme M, Carrondo-Cottin S, Valdès MM, Simonyan D, Audet MÈ, Gariépy JL, Camden MC, Gariépy C, Verreault S, Lavoie P (2022) Association between early ischemic changes and collaterals in acute stroke: a retrospective study. AJNR Am J Neuroradiol 43:1424–1430
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD, ESCAPE Trial Investigators (2015) Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372:1019–1030
Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, Poppe AY, Buck BH, Field TS, Dowlatshahi D, van Adel BA, Swartz RH, Shah RA, Sauvageau E, Zerna C, Ospel JM, Joshi M, Almekhlafi MA, Ryckborst KJ, Lowerison MW, Heard K, Garman D, Haussen D, Cutting SM, Coutts SB, Roy D, Rempel JL, Rohr AC, Iancu D, Sahlas DJ, Yu AYX, Devlin TG, Hanel RA, Puetz V, Silver FL, Campbell BCV, Chapot R, Teitelbaum J, Mandzia JL, Kleinig TJ, Turkel-Parrella D, Heck D, Kelly ME, Bharatha A, Bang OY, Jadhav A, Gupta R, Frei DF, Tarpley JW, McDougall CG, Holmin S, Rha JH, Puri AS, Camden MC, Thomalla G, Choe H, Phillips SJ, Schindler JL, Thornton J, Nagel S, Heo JH, Sohn SI, Psychogios MN, Budzik RF, Starkman S, Martin CO, Burns PA, Murphy S, Lopez GA, English J, Tymianski M, ESCAPE-NA1 Investigators (2020) Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet 395:878–887
Ospel JM, McDonough R, Demchuk AM, Menon BK, Almekhlafi MA, Nogueira RG, McTaggart RA, Poppe AY, Buck BH, Roy D, Haussen DC, Chapot R, Field TS, Jayaraman MV, Tymianski M, Hill MD, Goyal M, ESCAPE-NA1 investigators (2022) Predictors and clinical impact of infarct progression rate in the ESCAPE-NA1 trial. J Neurointerv Surg 14:886–891
Boers AMM, Sales Barros R, Jansen IGH, Berkhemer OA, Beenen LFM, Menon BK, Dippel DWJ, van der Lugt A, van Zwam WH, Roos YBWEM, van Oostenbrugge RJ, Slump CH, Majoie CBLM, Marquering HA, MR CLEAN investigators (2018) Value of quantitative collateral scoring on CT angiography in patients with acute ischemic stroke. AJNR Am J Neuroradiol 39:1074–1082
Wolff L, Su J, Van Loon D, van Es A, van Doormaal PJ, Majoie C, van Zwam W, Dippel D, van der Lugt A, van Walsum T, MR CLEAN investigators (2022) Inter-rater reliability for assessing intracranial collaterals in patients with acute ischemic stroke: comparing 29 raters and an artificial intelligence-based software. Neuroradiology 64:2277–2284
Grunwald IQ, Kulikovski J, Reith W, Gerry S, Namias R, Politi M, Papanagiotou P, Essig M, KawMathur S, Joly O, Hussain K, Wagner V, Shah S, Harston G, Vlahovic J, Walter S, Podlasek A, Fassbender K (2019) Collateral automation for triage in stroke: evaluating automated scoring of collaterals in acute stroke on computed tomography scans. Cerebrovasc Dis 47:217–222
Yang W, Soomro J, Jansen IGH, Venkatesh A, Yoo AJ, Lopes D, Beenen LFM, Emmer BJ, Majoie CBLM, Marquering HA (2022) Collateral capacity assessment: robustness and interobserver agreement of two grading scales and agreement with quantitative scoring. Clin Neuroradiol 26, Online ahead of print
Valente M, Bivard A, Cheung A, Manning NW, Parsons MW (2023) CT vascular territory mapping: a novel method to identify large vessel occlusion collateral. Neuroradiology 65:113–119
Lin Y, Kang N, Kang J, Lv S, Wang J (2019) Predictive value of time-variant color-coded multiphase CT angiography (mCTA) regarding clinical outcome of acute ischemic stroke: in comparison with conventional mCTA and CT perfusion. Acta Radiol 63:84–92
Ospel JM, Volny O, Qiu W, Najm M, Kashani N, Goyal M, Menon BK (2020) Displaying multiphase CT angiography using a time-variant color map: practical considerations and potential applications in patients with acute stroke. AJNR Am J Neuroradiol 41:200–205
Ospel JM, Cimflova P, Volny O, Qiu W, Hafeez M, Mayank A, Najm M, Chung K, Kashani N, Almekhlafi MA, Menon BK, Goyal M (2021) Utility of time-variant multiphase CTA color maps in outcome prediction for acute ischemic stroke due to anterior circulation large vessel occlusion
Su J, Wolff L, van Doormaal PJ, Dippel DWJ, van Zwam W, Niessen WJ, van der Lugt A, van Walsum T (2023) Time dependency of automated collateral scores in computed tomography angiography and computed tomography perfusion images in patients with intracranial arterial occlusion. Neuroradiology 65:313–322
Mortimer AM, Simpson E, Bradley MD, Renowden SA (2013) Computed tomography angiography in hyperacute ischemic stroke: prognostic implications and role in decision-making. Stroke 44:1480–1488
Leng X, Fang H, Leung TW, Mao C, Xu Y, Miao Z, Liu L, Wong KS, Liebeskind DS (2016) Impact of collateral status on successful revascularization in endovascular treatment: a systematic review and meta-analysis. Cerebrovasc Dis 41:27–34
Dong S, Yu C, Wu Q, Xia H, Xu J, Gong K, Wang T (2022) Predictors of symptomatic intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis 24:1–13
Menon BK, Qazi E, Nambiar V, Foster LD, Yeatts SD, Liebeskind D, Jovin TG, Goyal M, Hill MD, Tomsick TA, Broderick JP, Demchuk AM, Interventional Management of Stroke III Investigators (2015) Differential effect of baseline computed tomographic angiography collaterals on clinical outcome in patients enrolled in the interventional management of stroke III trial. Stroke 46:1239–1244
Berkhemer OA, Jansen IG, Beumer D, Fransen PS, van den Berg LA, Yoo AJ, Lingsma HF, Sprengers ME, Jenniskens SF, Lycklama Nijeholt ÀGJ, van Walderveen MA, van den Berg R, Bot JC, Beenen LF, Boers AM, Slump CH, Roos YB, van Oostenbrugge RJ, Dippel DW, van der Lugt A, van Zwam WH, Marquering HA, Majoie CB, MR CLEAN Investigators (2016) Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke 47:768–776
Liebeskind DS, Saber H, Xiang B, Jadhav AP, Jovin TG, Haussen DC, Budzik RF, Bonafe A, Bhuva P, Yavagal DR, Hanel RA, Ribo M, Cognard C, Sila C, Hassan AE, Smith WS, Saver JL, Nogueira RG, DAWN Investigators (2022) Collateral circulation in thrombectomy for stroke after 6 to 24 hours in the DAWN trial. Stroke 53:742–748
Jansen IG, Mulder MJ, Goldhoorn RB, Boers AM, van Es AC, Yo LS, Hofmeijer J, Martens JM, van Walderveen MA, van der Kallen BF, Jenniskens SF, Treurniet KM, Marquering HA, Sprengers ME, Schonewille WJ, Bot JC, Lycklama Nijeholt AGJ, Lingsma HF, Liebeskind DS, Boiten J, Vos JA, Roos YB, van Oostenbrugge RJ, van der Lugt A, van Zwam WH, Dippel DW, van den Wijngaard IR, Majoie CB, MR CLEAN Registry investigators (2019) Impact of single phase CT angiography collateral status on functional outcome over time: results from the MR CLEAN Registry. J Neurointerv Surg 11:866–873
Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CBLM, Campbell BCV, Guillemin F, Lingsma H, Anxionnat R, Epstein J, Saver JL, Marquering H, Wong JH, Lopes D, Reimann G, Desal H, Dippel DWJ, Coutts S, du Mesnil de Rochemont R, Yavagal D, Ferre JC, Roos YBWEM, Liebeskind DS, Lenthall R, Molina C, Al Ajlan FS, Reddy V, Dowlatshahi D, Sourour NA, Oppenheim C, Mitha AP, Davis SM, Weimar C, van Oostenbrugge RJ, Cobo E, Kleinig TJ, Donnan GA, van der Lugt A, Demchuk AM, Berkhemer OA, Boers AMM, Ford GA, Muir KW, Brown BS, Jovin T, van Zwam WH, Mitchell PJ, Hill MD, White P, Bracard S, Goyal M, HERMES collaborators (2018) Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol 17:895–904
de Havenon A, Mlynash M, Kim-Tenser MA, Lansberg MG, Leslie-Mazwi T, Christensen S, McTaggart RA, Alexander M, Albers G, Broderick J, Marks MP, Heit JJ, DEFUSE 3 Investigators (2019) Results from DEFUSE 3: good collaterals are associated with reduced ischemic core growth but not neurologic outcome. Stroke 50:632–638
Kim YD, Nam HS, Yoo J, Park H, Sohn SI, Hong JH, Kim BM, Kim DJ, Bang OY, Seo WK, Chung JW, Lee KY, Jung YH, Lee HS, Ahn SH, Shin DH, Choi HY, Cho HJ, Baek JH, Kim GS, Seo KD, Kim SH, Song TJ, Kim J, Han SW, Park JH, Lee SI, Heo J, Choi JK, Heo JH, Thrombus Imaging Study Group and the SECRET Study Group (2021) Prediction of early recanalization after intravenous thrombolysis in patients with large-vessel occlusion. J Stroke 23:244–252
García-Tornel Á, Ciolli L, Rubiera M, Requena M, Muchada M, Pagola J, Rodriguez-Luna D, Deck M, Juega J, Rodríguez-Villatoro N, Boned S, Olivé-Gadea M, Sanjuan E, Tomasello A, Piñana C, Hernández D, Álvarez-Sabin J, Molina CA, Ribó M (2021) Leptomeningeal collateral flow modifies endovascular treatment efficacy on large-vessel occlusion strokes. Stroke 52:299–303
Uniken Venema SM, Wolff L, van den Berg SA, Reinink H, Luijten SPR, Lingsma HF, Marquering HA, Boers AMM, Bot J, Hammer S, Nederkoorn PJ, Roos YBWEM, Majoie CBLM, Dankbaar JW, van der Lugt A, van der Worp HB, MRCLEAN Registry Investigators (2022) Time since stroke onset, quantitative collateral score, and functional outcome after endovascular treatment for acute ischemic stroke. Neurology 99:e1609–e1618
Kim BM, Baek JH, Heo JH, Nam HS, Kim YD, Yoo J, Kim DJ, Jeon P, Baik SK, Suh SH, Lee KY, Kwak HS, Roh HG, Lee YJ, Kim SH, Ryu CW, Ihn YK, Kim B, Jeon HJ, Kim JW, Byun JS, Suh S, Park JJ, Lee WJ, Roh J, Shin BS, Bang OY (2018) Collateral status affects the onset-to-reperfusion time window for good outcome. J Neurol Neurosurg Psychiatry 89:903–909
Uniken Venema SM, Dankbaar JW, Wolff L, van Es ACGM, Sprengers M, van der Lugt A, Dippel DWJ, van der Worp HB; MR CLEAN Registry investigators (2022) Collateral status and recanalization after endovascular treatment for acute ischemic stroke. J Neurointerv Surg 18, Online ahead of print
Boers AM, Jansen IG, Berkhemer OA, Yoo AJ, Lingsma HF, Slump CH, Roos YB, van Oostenbrugge RJ, Dippel DW, van der Lugt A, van Zwam WH, Marquering HA, Majoie CB, MR CLEAN trial investigators (2017) Collateral status and tissue outcome after intra-arterial therapy for patients with acute ischemic stroke. J Cereb Blood Flow Metab 37:3589–3598
Al-Dasuqi K, Payabvash S, Torres-Flores GA, Strander SM, Nguyen CK, Peshwe KU, Kodali S, Silverman A, Malhotra A, Johnson MH, Matouk CC, Schindler JL, Sansing LH, Falcone GJ, Sheth KN, Petersen NH (2020) Effects of collateral status on infarct distribution following endovascular therapy in large vessel occlusion stroke. Stroke 51:e193–e202
van Kranendonk KR, Treurniet KM, Boers AMM, Berkhemer OA, van den Berg LA, Chalos V, Lingsma HF, van Zwam WH, van der Lugt A, van Oostenbrugge RJ, Dippel DWJ, Roos YBWEM, Marquering HA, Majoie CBLM, MR CLEAN Investigators (2019) Clinical and imaging markers associated with hemorrhagic transformation in patients with acute ischemic Stroke. Stroke 50:2037–2043
van der Steen W, van der Ende NAM, van Kranendonk KR, Chalos V, van Oostenbrugge RJ, van Zwam WH, Roos YBWEM, van Doormaal PJ, van Es ACGM, Lingsma HF, Majoie CBLM, van der Lugt A, Dippel DWJ, Roozenbeek B, MR CLEAN Trial MR CLEAN Registry Investigators (2022) Determinants of symptomatic intracranial hemorrhage after endovascular stroke treatment: a retrospective cohort study. Stroke 53:2818–2827
Vagal A, Aviv R, Sucharew H, Reddy M, Hou Q, Michel P, Jovin T, Tomsick T, Wintermark M, Khatri P (2018) Collateral clock is more important than time clock for tissue fate. Stroke 49:2102–2107
Renú A, Laredo C, Montejo C, Zhao Y, Rudilosso S, Macias N, Llull L, Zarco F, Amaro S, Werner M, Obach V, Macho J, Chamorro A, Urra X (2019) Greater infarct growth limiting effect of mechanical thrombectomy in stroke patients with poor collaterals. J Neurointerv Surg 11:989–993
Jiang B, Ball RL, Michel P, Li Y, Zhu G, Ding V, Su B, Naqvi Z, Eskandari A, Desai M, Wintermark M (2019) Factors influencing infarct growth including collateral status assessed using computed tomography in acute stroke patients with large artery occlusion. Int J Stroke 14:603–612
Seo WK, Liebeskind DS, Yoo B, Sharma L, Jahan R, Duckwiler G, Tateshima S, Nour M, Szeder V, Colby G, Starkman S, Rao N, Bahr Hosseini M, Saver JL, UCLA Penumbra Imaging Investigators (2020) Predictors and functional outcomes of fast, intermediate, and slow progression among patients with acute ischemic stroke. Stroke 51:2553–2557
Boulouis G, Lauer A, Siddiqui AK, Charidimou A, Regenhardt RW, Viswanathan A, Rost N, Leslie-Mazwi TM, Schwamm LH (2017) Clinical imaging factors associated with infarct progression in patients with ischemic stroke during transfer for mechanical thrombectomy. JAMA Neurol 74:1361–1367
Puhr-Westerheide D, Tiedt S, Rotkopf LT, Herzberg M, Reidler P, Fabritius MP, Kazmierczak PM, Kellert L, Feil K, Thierfelder KM, Dorn F, Liebig T, Wollenweber FA, Kunz WG (2019) Clinical and imaging parameters associated with hyperacute infarction growth in large vessel occlusion stroke. Stroke 50:2799–2804
Agarwal S, Bivard A, Warburton E, Parsons M, Levi C (2018) Collateral response modulates the time-penumbra relationship in proximal arterial occlusions. Neurology 90:e316–e322
Nannoni S, Cereda CW, Sirimarco G, Lambrou D, Strambo D, Eskandari A, Dunet V, Wintermark M, Michel P (2019) Collaterals are a major determinant of the core but not the penumbra volume in acute ischemic stroke. Neuroradiology 61:971–978
Alves HC, Treurniet KM, Dutra BG, Jansen IGH, Boers AMM, Santos EMM, Berkhemer OA, Dippel DWJ, van der Lugt A, van Zwam WH, van Oostenbrugge RJ, Lingsma HF, Roos YBWEM, Yoo AJ, Marquering HA, Majoie CBLM, MR CLEAN trial investigators (2018) Associations between collateral status and thrombus characteristics and their impact in anterior circulation stroke. Stroke 49:391–396
Qazi EM, Sohn SI, Mishra S, Almekhlafi MA, Eesa M, d’Esterre CD, Qazi AA, Puig J, Goyal M, Demchuk AM, Menon BK (2015) Thrombus characteristics are related to collaterals and angioarchitecture in acute stroke. Can J Neurol Sci 42:381–388
Broocks G, Kemmling A, Meyer L, Nawabi J, Schön G, Fiehler J, Kniep H, Hanning U (2019) Computed tomography angiography collateral profile is directly linked to early edema progression rate in acute ischemic stroke. Stroke 50:3424–3430
Broocks G, Kniep H, Schramm P, Hanning U, Flottmann F, Faizy T, Schönfeld M, Meyer L, Schön G, Aulmann L, Machner B, Royl G, Fiehler J, Kemmling A (2020) Patients with low Alberta stroke program early CT score (ASPECTS) but good collaterals benefit from endovascular recanalization. J Neurointerv Surg 12:747–752
Seker F, Pereira-Zimmermann B, Pfaff J, Purrucker J, Gumbinger C, Schönenberger S, Bendszus M, Möhlenbruch MA (2020) Collateral scores in acute ischemic Stroke: a retrospective study assessing the suitability of collateral scores as standalone predictors of clinical outcome. Clin Neuroradiol 30:789–793
Gensicke H, Al-Ajlan F, Fladt J, Campbell BCV, Majoie CBLM, Bracard S, Hill MD, Muir KW, Demchuk A, San Román L, van der Lugt A, Liebeskind DS, Brown S, White PM, Guillemin F, Dávalos A, Jovin TG, Saver JL, Dippel DWJ, Goyal M, Mitchell PJ, Menon BK, HERMES Collaborators (2022) Comparison of three scores of collateral status for their association with clinical outcome: the HERMES collaboration. Stroke 53:3548–3556
Kauw F, Heit JJ, Martin BW, van Ommen F, Kappelle LJ, Velthuis BK, de Jong HWAM, Dankbaar JW, Wintermark M (2018) Collateral status in ischemic stroke: a comparison of CT angiography, CT perfusion and digital subtraction angiography. J Comput Assist Tomogr 44:984–992
Reid M, Famuyide AO, Forkert ND, Sahand Talai A, Evans JW, Sitaram A, Hafeez M, Najm M, Menon BK, Demchuk A, Goyal M, Sah RG, d’Esterre CD, Barber P (2019) Accuracy and reliability of multiphase CTA perfusion for identifying ischemic core. Clin Neuroradiol 29:543–552
d’Esterre CD, Trivedi A, Pordeli P, Boesen M, Patil S, Hwan Ahn S, Najm M, Fainardi E, Shankar JJ, Rubiera M, Almekhlafi MA, Mandzia J, Khaw AV, Barber P, Coutts S, Hill MD, Demchuk AM, Sajobi T, Forkert ND, Goyal M, Lee TY, Menon BK (2017) Regional comparison of multiphase computed tomographic angiography and computed tomographic perfusion for prediction of tissue fate in ischemic stroke. Stroke 48:939–945
Parthasarathy R, Kate M, Rempel JL, Liebeskind DS, Jeerakathil T, Butcher KS, Shuaib A (2013) Prognostic evaluation based on cortical vein score difference in stroke. Stroke 44:2748–2754
Bhaskar S, Bivard A, Parsons M, Nilsson M, Attia JR, Stanwell P, Levi C (2017) Delay of late-venous phase cortical vein filling in acute ischemic stroke patients: associations with collateral status. J Cereb Blood Flow Metab 37:671–682
Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis GM, Broocks G, Flottmann F, Marks MP, Lansberg MG, Albers GW, Fiehler J, Wintermark M, Heit JJ (2021) Favorable venous outflow profiles correlate with favorable tissue-level collaterals and clinical outcome. Stroke 52:1761–1767
Jansen IGH, van Vuuren AB, van Zwam WH, van den Wijngaard IR, Berkhemer OA, Lingsma HF, Slump CH, van Oostenbrugge RJ, Treurniet KM, Dippel DWJ, van Walderveen MAA, van der Lugt A, Roos YBWEM, Marquering HA, Majoie CBLM, van den Berg R, Trial Investigators MRCLEAN (2018) Absence of cortical vein opacification is associated with lack of intra-arterial therapy benefit in stroke. Radiology 286:731
Hoffman H, Ziechmann R, Swarnkar A, Masoud HE, Gould G (2019) Cortical vein opacification for risk stratification in anterior circulation endovascular thrombectomy. J Stroke Cerebrovasc Dis 28:1710–1717
Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis G, Meyer L, Marks MP, Broocks G, Flottmann F, Lansberg MG, Albers GW, Fiehler J, Wintermark M, Heit JJ (2021) Venous outflow profiles are linked to cerebral edema formation at noncontrast head CT after treatment in acute ischemic stroke regardless of collateral vessel status at CT angiography. Radiology 299:682–690
Faizy TD, Kabiri R, Christensen S, Mlynash M, Kuraitis G, Mader MM, Albers GW, Lansberg MG, Fiehler J, Wintermark M, Marks MP, Heit JJ (2021) Association of venous outflow profiles and successful vessel reperfusion after thrombectomy. Neurology 96:e2903-2911
van Horn N, Heit JJ, Kabiri R, Broocks G, Christensen S, Mlynash M, Meyer L, Schoenfeld MH, Lansberg MG, Albers GW, Fiehler J, Wintermark M, Faizy TD (2022) Venous outflow profiles are associated with early edema progression in ischemic stroke. Int J Stroke 17:1078–1084
Faizy TD, Mlynash M, Marks MP, Christensen S, Kabiri R, Kuraitis GM, Broocks G, Winkelmeier L, Geest V, Nawabi J, Lansberg MG, Albers GW, Fiehler J, Wintermark M, Heit JJ (2022) Intravenous tPA (tissue-Type Plasminogen Activator) correlates with favorable venous outflow profiles in acute ischemic stroke. Stroke 53:3145–3152
Winkelmeier L, Heit JJ, Adusumilli G, Geest V, Guenego A, Broocks G, Prüter J, Gloyer NO, Meyer L, Kniep H, Lansberg MG, Albers GW, Wintermark M, Fiehler J, Faizy TD (2023) Poor venous outflow profiles increase the risk of reperfusion hemorrhage after endovascular treatment. J Cereb Blood Flow Metab 43:72–83
Singh N, Bala F, Kim BJ, Najm M, Ahn SH, Fainardi E, Rubiera M, Khaw AV, Zini A, Goyal M, Menon BK, Almekhlafi M (2022) Time-resolved assessment of cortical venous drainage on multiphase CT angiography in patients with acute ischemic stroke. Neuroradiology 64:897–903
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG, DEFUSE 3 Investigators (2018) Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378:708–718
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG, Trial Investigators DAWN (2018) Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 378:11–21
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG, HERMES collaborators (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387:1723–1731
Almekhlafi MA, Kunz WG, McTaggart RA, Jayaraman MV, Najm M, Ahn SH, Fainardi E, Rubiera M, Khaw AV, Zini A, Hill MD, Demchuk AM, Goyal M, Menon BK (2020) Imaging triage of patients with late-window (6–24 hours) acute ischemic stroke: a comparative study using multiphase CT angiography versus CT perfusion. AJNR Am J Neuroradiol 41:129–133
Kim B, Jung C, Nam HS, Kim BM, Kim YD, Heo JH, Kim DJ, Kim JH, Han K, Kim JH, Kim BJ (2019) Comparison between perfusion- and collateral-based triage for endovascular thrombectomy in a late time window. Stroke 50:3465–3470
Menon BK, Ospel JM, McTaggart RA, Nogueira RG, Demchuk AM, Poppe A, Rempel JL, Zerna C, Joshi M, Almekhlafi MA, Field TS, Dowlatshahi D, van Adel BA, Sauvageau E, Tarpley J, Moreira T, Bang OY, Heck D, Psychogios MN, Tymianski M, Hill MD, Goyal M, ESCAPE-NA1 investigators (2020) Imaging criteria across pivotal randomized controlled trials for late window thrombectomy patient selection. J NeuroInterv Surg 25, Online ahead of print
Almekhlafi MA, Thornton J, Casetta I, Goyal M, Nannoni S, Herlihy D, Fainardi E, Power S, Saia V, Hegarty A, Pracucci G, Demchuk A, Mangiafico S, Boyle K, Michel P, Bala F, Gill R, Kuczynski A, Ademola A, Hill MD, Toni D, Murphy S, Kim BJ, Menon BK, Selection Of Late-window Stroke for Thrombectomy by Imaging Collateral Extent (SOLSTICE) Consortium (2022) Stroke imaging prior to thrombectomy in the late window: results from a pooled multicentre analysis. J Neurol Neurosurg Psychiatry 93:468–474
Tan Z, Parsons M, Bivard A, Sharma G, Mitchell P, Dowling R, Bush S, Churilov L, Xu A, Yan B (2022) Comparison of computed tomography perfusion and multiphase computed tomography angiogram in predicting clinical outcomes in endovascular thrombectomy. Stroke 53:2926–2934
Ospel JM, Volny O, Qiu W, Najm M, Hafeez M, Abdalrahman S, Fainardi E, Rubiera M, Khaw A, Shankar JJ, Hill MD, Almekhlafi MA, Demchuk AM, Goyal M, Menon BK (2021) Impact of multiphase computed tomography angiography for endovascular treatment decision-making on outcomes in patients with acute ischemic stroke. J Stroke 23:377–387
Qiu W, Kuang H, Ospel JM, Hill MD, Demchuk AM, Goyal M, Menon BK (2021) Automated prediction of ischemic brain tissue fate from multiphase computed tomographic angiography in patients with acute ischemic stroke using machine learning. J Stroke 23:234–243
McDonough RV, Qiu W, Ospel JM, Menon BK, Cimflova P, Goyal M (2022) Multiphase CTA-derived tissue maps aid in detection of medium vessel occlusions. Neuroradiology 64:887–896
Faizy TD, Heit JJ (2021) Rethinking the collateral vasculature assessment in acute ischemic stroke: the comprehensive collateral cascade
Faizy TD, Mlynash M, Kabiri R, Christensen S, Kuraitis GM, Mader MM, Flottmann F, Broocks G, Lansberg MG, Albers GW, Marks MP, Fiehler J, Wintermark M, Heit JJ (2022) The cerebral collateral cascade: comprehensive blood flow in ischemic stroke. Top Magn Reson Imaging 30:181–186
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. None.
Author information
Authors and Affiliations
Contributions
GB and EF designed, wrote and reviewed this article. All authors reviewed this article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Busto, G., Morotti, A., Carlesi, E. et al. Pivotal role of multiphase computed tomography angiography for collateral assessment in patients with acute ischemic stroke. Radiol med 128, 944–959 (2023). https://doi.org/10.1007/s11547-023-01668-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-023-01668-9