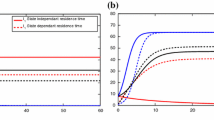
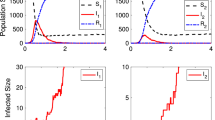
Abstract
This paper examines the short-term or transient dynamics of SIR infectious disease models in patch environments. We employ reactivity of an equilibrium and amplification rates, concepts from ecology, to analyze how dispersals/travels between patches, spatial heterogeneity, and other disease-related parameters impact short-term dynamics. Our findings reveal that in certain scenarios, due to the impact of spatial heterogeneity and the dispersals, the short-term disease dynamics over a patch environment may disagree with the long-term disease dynamics that is typically reflected by the basic reproduction number. Such an inconsistence can mislead the public, public healthy agencies and governments when making public health policy and decisions, and hence, these findings are of practical importance.







Similar content being viewed by others
References
Allen LJ, Bolker BM, Lou Y, Nevai AL (2007) Asymptotic profiles of the steady states for an SIS epidemic patch model. SIAM J Appl Math 67(5):1283–1309
Almarashi RM, McCluskey CC (2019) The effect of immigration of infectives on disease-free equilibria. J Math Biol 79:1015–1028
Anderson KE, Nisbet RM, McCauley E (2008) Transient responses to spatial perturbations in advective systems. Bull Math Biol 70:1480–1502
Andreasen V (2011) The final size of an epidemic and its relation to the basic reproduction number. Bull Math Biol 73:2305–2321
Arino J, Van den Driessche P (2003) A multi-city epidemic model. Math Popul Stud 10(3):175–193
Arino J, Van den Driessche P (2006) Disease spread in metapopulations. Fields Inst Commun 48:1–12
Arino J, Van Den Driessche P (2003) The basic reproduction number in a multi-city compartmental epidemic model. In: Positive systems. Springer, Berlin, pp 135–142
Chen S, Shi J, Shuai Z, Wu Y (2020) Asymptotic profiles of the steady states for an SIS epidemic patch model with asymmetric connectivity matrix. J Math Biol 80:2327–2361
Chepyzhov VV, Vishik MI (2002) Attractors for equations of mathematical physics. American Mathematical Society
Diekmann O, Heesterbeek JAP, Metz JA (1990) On the definition and the computation of the basic reproduction ratio \(R_0\) in models for infectious diseases in heterogeneous populations. J Math Biol 28:365–382
Eisenberg MC, Shuai Z, Tien JH, Van den Driessche P (2013) A cholera model in a patchy environment with water and human movement. Math Biosci 246:105–112
Harrington PD, Lewis MA, van den Driessche P (2022) Reactivity, attenuation, and transients in metapopulations. SIAM J Appl Dyn Syst 21:1287–1321
Hastings A (2001) Transient dynamics and persistence of ecological systems. Ecol Lett 4:215–220
Hastings A, Higgins K (1994) Persistence of transients in spatially structured ecological models. Science 263:1133–1136
Hethcote HW (1976) Qualitative analyses of communicable disease models. Math Biosci 28(3–4):335–356
Horn RA, Johnson CR (1985) Matrix analysis. Cambridge University Press, Cambridge
Hosack GR, Rossignol PA, Van Den Driessche P (2008) The control of vector-borne disease epidemics. J Theor Biol 255:16–25
Hsieh YH, Van den Driessche P, Wang L (2007) Impact of travel between patches for spatial spread of disease. Bull Math Biol 69:1355–1375
Kermack WO, McKendrick AG (1927) A contribution to the mathematical theory of epidemics. Proc R Soc Lond Ser A 115(772):700–721
Lutscher F, Wang X (2020) Reactivity of communities at equilibrium and periodic orbits. J Theor Biol 493:110240
Ma J, Earn DJD (2006) Generality of the final size formula for an epidemic of a newly invading infectious disease. Bull Math Biol 68:679–702
Mari L, Casagrandi R, Rinaldo A, Gatto M (2017) A generalized definition of reactivity for ecological systems and the problem of transient species dynamics. Methods Ecol Evol 8:1574–1584
Mari L, Casagrandi R, Rinaldo A, Gatto M (2018) Epidemicity thresholds for water-borne and water-related diseases. J Theor Biol 447:126–138
Mari L, Casagrandi R, Bertuzzo E, Rinaldo A, Gatto M (2019) Conditions for transient epidemics of waterborne disease in spatially explicit systems. R Soc Open Sci 6:181517
Mari L, Casagrandi R, Bertuzzo E, Pasetto D, Miccoli S, Rinaldo A, Gatto M (2021) The epidemicity index of recurrent SARS-CoV-2 infections. Nat Commun 12:2752
Neubert MG, Caswell H (1997) Alternatives to resilience for measuring the responses of ecological systems to perturbations. Ecology 78(3):653–665
O’Regan SM, O’Dea EB, Rohani P, Drake JM (2020) Transient indicators of tipping points in infectious diseases. J R Soc Interface 17:20200094
Ruxton GD, Doebeli M (1996) Spatial self-organization and persistence of transients in a metapopulation model. Proc R Soc Lond B 263:1153–1158
Saravia LA, Ruxton GD, Coviella CE (2000) The importance of transient’s dynamics in spatially extended populations. Proc R Soc Lond B 267:1781–1785
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180:29–48
Wang W, Zhao XQ (2004) An epidemic model in a patchy environment. Math Biosci 190:97–112
Wang X, Efendiev M, Lutscher F (2019) How spatial heterogeneity affects transient behavior in reaction-diffusion systems for ecological interactions? Bull Math Biol 81:3889–3917
Woodall H, Bullock JM, White SM (2014) Modelling the harvest of an insect pathogen. Ecol Model 287:16–26
Acknowledgements
The authors are grateful to the two anonymous referees for their careful reading and valuable comments on the first version of the paper, which have led to a significant improvement in readability of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Research partially supported by NSERC of Canada (No. RGPIN-2022-04744).
Appendix
Appendix
1.1 The Dependence of \(\lambda _{\max }\) and \(\lambda _{\min }\) for SIR Epidemic Patch Model
Taking partial derivatives of (11) with respect to \(\Gamma _0^{(i)}\) for \(i \in \{1, \, 2\}\), we have
where \(M:=m_1-m_2=\Gamma _0^{(1)}-d_{21}^I-\Gamma _0^{(2)}+d_{12}^I\) and \(D=d_{12}^I+d_{21}^I>0\). All of these partial derivatives are positive since
With respect to travel rates, the partial derivative of \(\lambda _{\max }\),
is positive if \(M<0\) and is negative if \(M>0\), and,
is positive if \(M>0\) and is negative if \(M<0\). As for \(\lambda _{\min }\), the partial derivatives are
which are both negative by the inequality (17).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, A., Zou, X. \(R_0\) May Not Tell Us Everything: Transient Disease Dynamics of Some SIR Models Over Patchy Environments. Bull Math Biol 86, 41 (2024). https://doi.org/10.1007/s11538-024-01271-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-024-01271-7