Abstract
Background
Atezolizumab plus bevacizumab has recently been approved as a new first-line standard of care for patients with unresectable hepatocellular carcinoma (HCC).
Objective
We performed a real-world study to evaluate the impact of the IMbrave150 trial inclusion criteria on the safety and efficacy of treatment outside of clinical trials.
Methods
We analyzed patients treated with atezolizumab plus bevacizumab for unresectable HCC from four different countries. No specific inclusion and exclusion criteria were applied, except for the absence of previous systemic therapies for HCC. The entire population was split into two groups according to concordance with the inclusion criteria as reported in the IMbrave150 trial in ‘IMbrave150-in’ and ‘IMbrave150-out’ patients, and safety and efficacy in the two groups of patients were evaluated.
Results
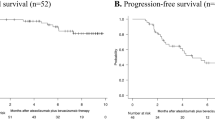
Overall, 766 patients were included in the analysis: 561/766 (73%) in the ‘IMbrave150-in’ group and 205/766 (27%) in the ‘IMbrave150-out’ group. Median overall survival (OS) and median progression-free survival (PFS) were 16.3 versus 14.3 months (hazard ratio [HR] 0.48, 95% confidence interval [CI] 0.35–0.65; p < 0.0001] and 8.3 versus 6.0 months (HR 0.79, 95% CI 0.63–0.99; p = 0.0431) in ‘IMbrave150-in’ and ‘IMbrave150-out’ patients, respectively. Multivariate analysis confirmed that patients included in the ‘IMbrave150-in’ group had significantly longer OS compared with patients included in the ‘IMbrave150-out’ group (HR 0.76, 95% CI 0.47–0.97; p = 0.0195). In ‘IMbrave150-in’ patients, the albumin-bilirubin (ALBI) grade was not associated with OS, whereas in ‘IMbrave150-out’ patients, those with ALBI grade 1 reported a significant benefit in terms of OS compared with those with ALBI grade 2 (16.7 vs. 5.9 months; HR 4.40, 95% CI 2.40–8.08; p > 0.0001). No statistically significant differences were reported in the ‘IMbrave150-in’ and ‘IMbrave150-out’ groups in terms of safety profile.
Conclusion
Adherence to the IMbrave150 trial inclusion criteria favorably impacts the prognosis of patients receiving atezolizumab plus bevacizumab. Among patients who did not meet the IMbrave150 inclusion criteria, those with ALBI grade 1 could benefit from the treatment.


Similar content being viewed by others
REFERENCES
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. https://doi.org/10.1038/s41572-020-00240-3.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. https://doi.org/10.1056/NEJMoa0708857.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. https://doi.org/10.1016/S1470-2045(08)70285-7.
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73.
Casadei-Gardini A, Rimini M, Kudo M, Shimose S, Tada T, Suda G, et al. REal life study of LEnVAtiNib therapy for hepatocellular carcinoma: RELEVANT study. Liver Cancer. 2022;11(6):527–39. https://doi.org/10.1159/000525145.
Rimini M, Shimose S, Lonardi S, Tada T, Masi G, Iwamoto H, et al. Lenvatinib versus Sorafenib as first-line treatment in hepatocellular carcinoma: a multi-institutional matched case-control study. Hepatol Res. 2021;51(12):1229–41. https://doi.org/10.1111/hepr.13718.
Casadei-Gardini A, Scartozzi M, Tada T, Yoo C, Shimose S, Masi G, et al. Lenvatinib versus sorafenib in first-line treatment of unresectable hepatocellular carcinoma: an inverse probability of treatment weighting analysis. Liver Int. 2021;41(6):1389–97. https://doi.org/10.1111/liv.14817.
Burgio V, Iavarone M, Di Costanzo GG, Marra F, Lonardi S, Tamburini E, et al. Real-life clinical data of lenvatinib versus sorafenib for unresectable hepatocellular carcinoma in Italy. Cancer Manag Res. 2021;13:9379–89. https://doi.org/10.2147/CMAR.S330195.
Rapposelli IG, Shimose S, Kumada T, Okamura S, Hiraoka A, Di Costanzo GG, et al. Identification of lenvatinib prognostic index via recursive partitioning analysis in advanced hepatocellular carcinoma. ESMO Open. 2021;6(4):100190. https://doi.org/10.1016/j.esmoop.2021.100190.
Rimini M, Kang W, Burgio V, Persano M, Aoki T, Shimose S, et al. Validation of the easy-to-use lenvatinib prognostic index to predict prognosis in advanced hepatocellular carcinoma patients treated with lenvatinib. Hepatol Res. 2022;52(12):1050–9. https://doi.org/10.1111/hepr.13824.
Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab As second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind. Phase III Trial J Clin Oncol. 2020;38(3):193–202. https://doi.org/10.1200/JCO.19.01307.
Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. https://doi.org/10.1016/S1470-2045(21)00604-5.
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. https://doi.org/10.1056/NEJMoa1915745.
Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab and durvalumab as first-line therapy in patients with unresectable hepatocellular carcinoma: HIMALAYA. J Clin Oncol. 2022;40(4 Suppl):379. https://doi.org/10.1200/JCO.2022.40.4_suppl.379-.
Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(8):995–1008. https://doi.org/10.1016/S1470-2045(22)00326-6.
Finn R, et al. Primary results from the phase 3 LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2022;33(Suppl 7):S808–69. https://doi.org/10.1016/annonc/annonc1089.
Qin S, et al. Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial. Ann Oncol. 2022;33(Suppl 7):S808–69. https://doi.org/10.1016/annonc/annonc1089.
Sonbol MB, Riaz IB, Naqvi SAA, Almquist DR, Mina S, Almasri J, et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: a systematic review and network meta-analysis. JAMA Oncol. 2020;6(12):e204930. https://doi.org/10.1001/jamaoncol.2020.4930.
Vogel A, Rimassa L, Sun HC, Abou-Alfa GK, El-Khoueiry A, Pinato DJ, et al. Comparative efficacy of atezolizumab plus bevacizumab and other treatment options for patients with unresectable hepatocellular carcinoma: a network meta-analysis. Liver Cancer. 2021;10(3):240–8. https://doi.org/10.1159/000515302.
Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–8. https://doi.org/10.1200/JCO.2014.57.9151.
Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies [in Spanish, English]. Actas Dermosifiliogr (Engl Ed). 2021;112(1):90–2. https://doi.org/10.1016/j.ad.2019.05.009.
D’Alessio A, Fulgenzi CAM, Nishida N, Schönlein M, von Felden J, Schulze K, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: A real-world study. Hepatology. 2022;76(4):1000–12. https://doi.org/10.1002/hep.32468.
Welland S, Leyh C, Finkelmeier F, Jefremow A, Shmanko K, Gonzalez-Carmona MA, et al. Real-world data for lenvatinib in hepatocellular carcinoma (ELEVATOR): a retrospective multicenter study. Liver Cancer. 2022;11(3):219–32. https://doi.org/10.1159/000521746.
Tovoli F, Ielasi L, Casadei-Gardini A, Granito A, Foschi FG, Rovesti G, et al. Management of adverse events with tailored sorafenib dosing prolongs survival of hepatocellular carcinoma patients. J Hepatol. 2019;71(6):1175–83. https://doi.org/10.1016/j.jhep.2019.08.015.
Sho T, Suda G, Yamamoto Y, Furuya K, Baba M, Ogawa K, et al. Efficacy and effect on liver functional reserve of atezolizumab and bevacizumab for Unresectable hepatocellular carcinoma in patients who do not meet eligibility criteria of IMbrave150. Cancers (Basel). 2022;14(16):3938. https://doi.org/10.3390/cancers14163938.
Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: The Need for a More Detailed Evaluation of Hepatic Function. Liver Cancer. 2017;6(4):325–36. https://doi.org/10.1159/000479984.
Rimini M, Rovesti G, Casadei-Gardini A. Child Pugh and ALBI grade: past, present or future? Ann Transl Med. 2020;8(17):1044. https://doi.org/10.21037/atm-20-3709.
de Castro T, Jochheim LS, Bathon M, Welland S, Scheiner B, Shmanko K, et al. Atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma with impaired liver function and prior systemic therapy: a real-world experience. Ther Adv Med Oncol. 2022;14:17588359221080298. https://doi.org/10.1177/17588359221080298.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflicts of interest
Lorenza Rimassa reports receiving consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, BMS, Celgene, Eisai, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Lilly, MSD, Nerviano Medical Sciences, Roche, Sanofi, Servier, Taiho Oncology, and Zymeworks; lecture fees from AbbVie, Amgen, Bayer, Eisai, Gilead, Incyte, Ipsen, Lilly, Merck Serono, Roche, Sanofi, and Servier; travel expenses from AstraZeneca; and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, and Zymeworks. Tiziana Pressiani has received consulting fees from Bayer, Ipsen, and IQVIA, and institutional research funding from Bayer, Lilly, and Roche. Fabian Finkelmeier has received travel support from Ipsen, and speaker’s fees from AbbVie, MSD, Ipsen, Eisai, and Fresenius. Mario Scartozzi has received grants and personal fees from MERCK, MSD, Servier, Eisai, and Amgen. Andrea Casadei-Gardini has received grants and personal fees from MSD, Eisai, and Bayer, and is an advisor for MSD, Eisai, Bayer, Bristol-Myers Squibb, AstraZeneca, and GSK. Margherita Rimini, Mara Persano , Toshifumi Tada, Goki Suda, Shigeo Shimose, Masatoshi Kudo, Jaekyung Cheon, Ho Yeong Lim, José Presa, Gianluca Masi, Changhoon Yoo, Sara Lonardi, Fabio Piscaglia, Takashi Kumada, Naoya Sakamoto, Hideki Iwamoto, Tomoko Aoki, Hong Jae Chon, Vera Himmelsbach, Margarida Montes, Caterina Vivaldi, Caterina Soldà, Atsushi Hiraoka, Takuya Sho, Takashi Niizeki, Naoshi Nishida, Christoph Steup, Masashi Hirooka, Kazuya Kariyama, Joji Tani, Masanori Atsukawa, Koichi Takaguchi, Ei Itobayashi, Shinya Fukunishi, Kunihiko Tsuji, Toru Ishikawa, Kazuto Tajiri, Hironori Ochi, Satoshi Yasuda, Hidenori Toyoda, Chikara Ogawa, Takashi Nishimura, Takeshi Hatanaka, Satoru Kakizaki, Noritomo Shimada, Kazuhito Kawata, Fujimasa Tada, Hideko Ohama, Kazuhiro Nouso, Asahiro Morishita, Akemi Tsutsui, Takuya Nagano, Norio Itokawa, Tomomi Okubo, Taeang Arai, Michitaka Imai, Hisashi Kosaka, Atsushi Naganuma, Yohei Koizumi, Shinichiro Nakamura, Masaki Kaibori, Hiroko Iijima, Yoichi Hiasa, Valentina Burgio, and Stefano Cascinu declare they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
The Ethical Review Board of each Institutional Hospital approved the present study. This study was performed in line with the principles of the Declaration of Helsinki.
Consent to participate
Written informed consent for treatment was obtained for all patients.
Consent for publication
Not applicable.
Data availability statement
Data available on request from the authors.
Code availability
Not applicable.
Authors' contributions
Conception and design: ACG, MR. Acquisition of data (acquired and managed patients): All authors. Analysis and interpretation of data: ACG, MR. Writing, review, and/or revision of the manuscript: ACG, MR. Final approval of the manuscript: All authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rimini, M., Persano, M., Tada, T. et al. Real-World Data for Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma: How Does Adherence to the IMbrave150 Trial Inclusion Criteria Impact Prognosis?. Targ Oncol 18, 221–233 (2023). https://doi.org/10.1007/s11523-023-00953-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-00953-x