Abstract
Background
Neurotrophic tyrosine receptor kinase (NTRK) gene fusions are present across various tumor types with an estimated overall prevalence of less than 1%. Tropomyosin receptor kinase inhibitors (TRKis) block the constitutively activated tyrosine receptor kinase (TRK) fusion protein produced in cancers with NTRK gene fusions (NTRK+) from downstream signaling. Many treatment guidelines now include TRKis as first-line (1L) or subsequent treatment options for TRK fusion cancer.
Objective
This study aimed to assess treatment patterns subsequent to a finding of NTRK+ status among patients with TRK fusion cancer.
Patients and Methods
This was a one-time, retrospective, multi-site patient chart abstraction by oncology practices in the USA from June to September 2020. US medical oncologists from the Oncology Provider Extended Network (OPEN) who had treated patients with NTRK+ advanced/metastatic solid tumors abstracted information into electronic case report forms (eCRFs) for adult patients with advanced/metastatic solid tumors and a NTRK+ tumor test result with a known fusion partner. Data abstracted into eCRFs by oncologists included demographic, clinical, and treatment characteristics of patients with advanced/metastatic TRK fusion solid tumors. Responses were summarized using descriptive statistics. Median treatment durations across the lines of therapy were estimated by Kaplan-Meier time to discontinuation.
Results
A total of 19 medical oncologists abstracted data from 110 patient charts.
Median patient age at advanced/metastatic diagnosis was 62 years. The majority of patients were male (58.2%) and White (79.1%). Solid tumor types reported in at least 10% of the study cohort were lung (24.5%), cholangiocarcinoma (13.6%), pancreatic (10.9%), and colorectal (10.0%). Results for patients with hepatobiliary cancers (i.e., cholangiocarcinoma, pancreatic cancer, hepatocellular carcinoma) and colorectal cancer, and appendiceal cancer are also included. Median duration of 1L TRKi therapy was 16.8 months across all solid tumor types, whereas median duration of 1L was 5.6 months among patients receiving non-TRKi therapies (p = 0.017). Among the solid tumor types represented by at least 10% of the study population, median duration of 1L TRKi therapy was only reached in patients with pancreatic cancer (3.3 months). Median duration of TRKi in the second-line (2L) setting was 7.9 months overall, relative to 5.3 months among patients receiving non-TRKi therapies (p = 0.003). Across lung, cholangiocarcinoma, pancreatic, and colorectal cancers, the median durations of 2L TRKi therapy were 14.1, 6.0, 6.1, and 4.1 months, respectively.
Conclusion and Relevance
Among patients with advanced/metastatic TRK fusion solid tumors, medical oncologists reported that approximately two-thirds initiated a TRKi during the study period. Treatment with a TRKi was longer in duration compared to non-TRKi treatment in 1L and 2L therapy. Additional research is needed to gain insight into the association between early TRKi therapy initiation and clinical outcomes in the real-world setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Oncologists in this study reported that two-thirds of patients with tumors containing certain types of biomarkers, called tyrosine receptor kinase (TRK) fusions, received tropomyosin receptor kinase inhibitors (TRKis), which are treatments for TRK fusion cancers. |
Patients receiving TRKis received TRKi therapy for a longer duration than patients who received other treatments in the same line of therapy. |
Future research should examine reasons patients with TRK fusion cancers do not receive TRKis, and elucidate the association between early TRKi therapy initiation and clinical outcomes. |
1 Introduction
Neurotrophic tyrosine receptor kinase (NTRK) gene fusions have been observed at relatively high frequencies, as high as 90% or more, in rare tumors such as congenital infantile fibrosarcoma [1,2,3,4,5], congenital mesoblastic nephroma [4, 6], secretory breast carcinoma [7, 8], and mammary analogue secretory carcinoma (MASC) of the salivary gland [9,10,11]. Some tumor types have been reported to harbor tyrosine receptor kinase (TRK) fusions in up to 35% of cases, including thyroid cancer (5–15%) [12], glioma [13], sarcomas such as inflammatory myofibroblastic tumors [14], and Spitzoid neoplasms.
Common cancers have been reported to harbor TRK fusions as well, but in much lower frequencies, often < 1%. These more common tumors include non-small cell lung cancer, breast cancer (other than secretory cancer), colorectal and appendiceal cancer, pancreatic cancer, head, and neck cancer (other than MASC), sarcoma, melanoma, glioblastoma multiforme, cholangiocarcinoma, and gastrointestinal stromal tumors. Overall, these fusions may be implicated in approximately 1% of all solid tumor cancers [12, 15,16,17,18,19,20].
Tropomyosin receptor kinase inhibitors (TRKis) target TRK fusion cancer by blocking the constitutively activated tyrosine receptor kinase (TRK) fusion protein produced by an NTRK gene fusion [21]. The FDA approved larotrectinib [22] and entrectinib [23] for the treatment of TRK fusion cancer in both adult (larotrectinib and entrectinib: all ages) and pediatric (larotrectinib: all ages, entrectinib: patients aged ≥ 12 years) populations.
However, both FDA approvals were based on trials recruiting small numbers of patients in single-arm trials. In this context, additional research is needed to ascertain how oncologists determine eligibility for TRKi therapy. Improved understanding of treatment decision making may enhance communication of both trial and real-world evidence (RWE) research results. As TRKis are relatively new therapies, data on patients with TRK fusion cancer treated outside the clinical trial setting and outside of major cancer treatment centers are lacking.
The objective of this study was to assess treatment patterns subsequent to a finding of NTRK gene fusion among patients with TRK fusion cancer.
2 Methods
A group of US community-based medical oncologists from the Oncology Provider Extended Network (OPEN) who had treated patients with TRK fusion cancer, was invited to participate in a retrospective patient chart review study. The OPEN community comprises more than 7000 unique providers in oncology, hematology, and urology across the USA. Over 800 of these physicians have participated in OPEN real-world research since 2016. Prior to chart data abstraction, study materials (research protocol and eCRF) were submitted to an independent, central Institutional Review Board (Western Institutional Review Board [IRB]). The IRB determined that this study met the criteria for a waiver of authorization for use and disclosure of protected health information (PHI) and was exempt from IRB oversight. A waiver of informed consent was granted.
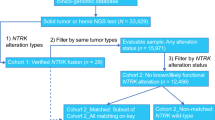
Medical oncologists from the OPEN community who had treated/managed adult patients with TRK fusion cancer and had previously ordered NTRK gene fusion testing between January 1, 2016, and December 30, 2019, by any testing modality (e.g., next-generation sequencing [NGS], fluorescent in situ hybridization studies [FISH]) abstracted retrospective, de-identified patient-level data. Each oncologist indicated solid tumor type for up to ten patients with diagnoses of TRK fusion advanced/metastatic solid tumors. The limit of ten eligible patients per provider was imposed to minimize potential bias from a single practice. Physicians were instructed to select the earliest patient meeting study selection criteria and to continue with identifying consecutive, eligible patients for the study period. Eligible patients were adults aged ≥ 18 years (given the composition of the physician network) with a confirmed diagnosis of any advanced/metastatic solid tumor between January 1, 2016, and December 31, 2019, and had an NTRK+ tumor test result with a known fusion partner. Patients were required to have at least 3 months of follow-up from the date of advanced/metastatic cancer diagnosis (unless deceased prior to 3 months following diagnosis) with all data to be abstracted from the medical chart available at the time of abstraction. Participating providers were required to have been the primary physician involved in eligible patients’ treatment decision making throughout the course of therapy and follow-up. Physicians were paid fair market value for their voluntary participation. Recruitment efforts were made to the entire OPEN community and targeted invitations to physicians who likely had patients who met the eligibility criteria based on prior feasibility assessments.
Patients with a prior primary cancer, diagnosed and treated within a year prior to first-line (1L) therapy initiation for the advanced/metastatic solid tumor of interest, as well as those participating in a clinical trial for treatment of their cancer were excluded. Data collection occurred from June through September 2020. Each completed eCRF was reviewed independently for implausible or inconsistent data. Providers were contacted with queries, and individual eCRFs that could not be validated were removed from the study dataset.
Responses regarding demographic, clinical characteristics, biomarker testing, and treatment patterns were abstracted from patient medical records into eCRFs. Providers were instructed to consult all available sources available to them to complete the eCRF. Results were summarized using descriptive statistics and stratified by tumor type as well as treatment type (received a TRKi vs did not receive a TRKi). Time to discontinuation of each line of therapy was estimated by the Kaplan-Meier method to account for right censoring of patients still on therapy at the time of last follow-up or time of data collection. All analyses were conducted in SAS v9.4.
3 Results
3.1 Physician Characteristics
Data from a total of 110 patient medical charts were entered by 19 providers during the data collection period. Data from all 110 patients were included in the final analytical cohort after completion of data validation. Medical oncologists from small (26.3%), medium (21.1%), and large (36.8%) community practices were represented, with 5.3% practicing as solo practitioners, and another 10.6% reporting an academic affiliation (Table 1). The participating medical oncologists reported a median of 17 years in practice. Approximately half (52.6%) practiced in urban settings, and the remainder practiced in suburban (31.6%) and rural (15.8%) settings. All four US regions were represented, with the South (36.8%) and West (31.6%) representing the greatest proportion of oncologists.
3.2 Patient Characteristics
Median patient age at advanced/metastatic diagnosis was 62 years (Table 2). The majority were male (58.2%) and White (79.1%). At advanced/metastatic diagnosis, over one-half of patients were commercially insured (56.4%), one-third (31.0%) of patients had Medicare, and 6.4%, 4.5%, and 0.9% were covered by multi-payers, Medicaid, or self-insured/self-pay, respectively. Over 90% of patients had at least 6 months of follow-up data. At the time of data collection, 62 (56.4%) patients were deceased, with a median time to death of 13 months from advanced/metastatic diagnosis. Tumor types reported at a frequency of 10% or greater (i.e., lung cancer, cholangiocarcinoma, pancreatic cancer, and colorectal cancer) are reported herein. Detailed results for other tumor types reported at a frequency of less than 10% can be found in supplemental material.
3.3 Tumor Type
3.3.1 Hepatobiliary Cancers
Among the 110 patients with an TRK fusion cancer, 33 (30.0%) had hepatobiliary cancers (i.e., cholangiocarcinoma, pancreatic cancer, or hepatocellular carcinoma), all 33 (100.0%) had stage IV cancer at initial diagnosis (Table 3). Nearly 80% of patients in the combined hepatobiliary cancer group had at least 6 months of follow-up from the date of advanced/metastatic diagnosis. After a median of 14.0 days from initial diagnosis, NTRK testing was ordered. A median of 2 lines of therapy was reported. Chemotherapy was the most commonly used 1L therapy with gemcitabine/cisplatin initiated as 1L therapy in 92.3%. Median duration of 1L therapy was 4.2 months. Among the 31 patients discontinuing 1L therapy, 25 discontinued 1L therapy due to disease progression (as defined clinically or confirmed with scan). Gemcitabine/cisplatin was used among 12 patients in 1L therapy. In 2L therapy, 4 patients had FOLFOX and 2 had gemcitabine/paclitaxel. In 3L therapy, 3 patients had entrectinib and 2 had larotrectinib. Median durations of 2L and 3L therapies across all patients were 6.4 months and 6.1 months, respectively (Table 3, Supplemental Table 1).
3.3.2 Lung Cancer
Among the 110 patients, 27 (24.5%) were diagnosed with lung cancer and 96.3% of the lung cancer patients were diagnosed with stage IV cancer at initial diagnosis (Table 3). Nearly 90% of the lung cancer patients included in the study had at least 6 months of follow-up from the date of advanced/metastatic diagnosis. After a median of 6 days from initial lung cancer diagnosis, NTRK testing was ordered. All patients received systemic therapy only after their advanced/metastatic diagnosis. The median number of lines of therapy was 2. Combination therapy was the most frequently reported regimen in the 1L setting among all lung cancer patients. First-line targeted therapy with entrectinib and larotrectinib was initiated by 3.7% and 7.4% of patients, respectively. Median duration of 1L therapy was 5.6 months among all lung cancer patients and 6.6 months among lung cancer patients with at least 6 months of follow-up data. Among those who discontinued 1L therapy (n = 20), 15 patients discontinued 1L due to disease progression (as confirmed with scan). Among the 17 patients treated with 2L therapy during the study period, targeted therapy was most frequently reported with entrectinib and larotrectinib initiated by 4 and 5 patients, respectively. Among the 4 patients treated with 3L therapy during the study period, larotrectinib was used by 2 (50.0%) patients. Median durations of 2L and 3L therapies across all patients were 5.6 and 3.3 months, respectively (Table 3, Supplemental Table 1).
3.3.3 Cholangiocarcinoma
There were 15 (13.6%) of the 110 patients who were diagnosed with cholangiocarcinoma, and all 15 (100%) were diagnosed with stage IV cancer at initial diagnosis (Table 3). Over three-fourths (80%) had at least 6 months of follow-up data from the date of advanced/metastatic diagnosis. After a median of 16 days from initial cholangiocarcinoma diagnosis, NTRK testing was ordered. All 15 patients received systemic therapy after their advanced/metastatic diagnosis with 13.3% also receiving best supportive care (BSC). The median number of lines of therapy was 2. Chemotherapy was used by over 90% of cholangiocarcinoma patients during 1L therapy. During 1L therapy, targeted therapy with larotrectinib was initiated by 1 patient. Median duration of 1L therapy among all cholangiocarcinoma patients and among cholangiocarcinoma patients with at least 6 months of follow-up data was 4.2 and 5.2 months, respectively. Among the 13 patients discontinuing 1L therapy, almost half (53.8%) discontinued due to disease progression (as confirmed with scan). Among the 11 patients treated with 2L therapy, targeted therapy was used by 8 patients, with 4 patients initiating entrectinib and 4 initiating larotrectinib. Among the 3 patients initiating 3L therapy, larotrectinib and entrectinib were used by 2 patients and 1 patient, respectively. Across both 2L and 3L, median durations of therapy were 6.1 months (Table 3, Supplemental Table 1).
3.3.4 Pancreatic Cancer
Twelve (10.9%) of the 110 patients had pancreatic cancer, and all (100%) were stage IV at initial diagnosis (Table 3). Three-fourths (75%) of pancreatic cancer patients included in the study had at least 6 months of follow-up from the date of advanced/metastatic diagnosis. After a median of 14.5 days from initial pancreatic cancer diagnosis, NTRK testing was ordered. Patients only received systemic therapy after their advanced/metastatic diagnosis. The median number of lines of therapy was 2. Chemotherapy was the most commonly (83.3%) used 1L therapy among pancreatic cancer patients and larotrectinib was initiated as 1L therapy in 16.7% of the patients. Median duration of 1L therapy among pancreatic cancer patients and among those with at least 6 months of follow-up data was 4.7 and 5.3 months, respectively. Among the 12 patients discontinuing 1L therapy, 11 discontinued due to disease progression (as defined clinically or confirmed with scan). The use of targeted therapy increased from 2 patients during 1L therapy to 4 during 2L therapy. During 3L therapy, 2 patients initiated entrectinib. Median durations of 2L and 3L therapies across all patients were 7.7 months and not reached, respectively (Table 3, Supplemental Table 1).
3.3.5 Colorectal Cancer + Appendiceal Cancer
Of the 110 patients with colorectal or appendiceal cancer, 13 (11.8%) had advanced/metastatic cancer and 84.6 of the 13 were diagnosed with stage IV cancer at initial diagnosis (Table 3). Neurotrophic tyrosine receptor kinase testing was ordered after a median of 7 days from initial diagnosis. All patients received systemic therapy after their advanced/metastatic diagnosis with 1 patient receiving both systemic therapy and BSC. The median number of lines of therapy was 2. FOLFOX (23.1%) and FOLFOX/bevacizumab (15.4%) were common 1L regimens. Median duration of 1L therapy was 6.7 months. Among the 10 patients discontinuing 1L therapy, 7 discontinued due to disease progression (as confirmed with scan), with the other 3 patients discontinuing due to completion of scheduled duration of therapy. Among the 10 patients treated with 2L therapy, 3 received larotrectinib and 3 received folinic acid, fluorouracil, and irinotecan (FOLFIRI). Among the 5 patients who had 3L therapy, 2 received larotrectinib and 2 received regorafenib. Median durations of 2L and 3L therapies across all patients were 4.6 and 6.0 months, respectively (Table 3, Supplemental Table 1).
3.4 Therapy Type
3.4.1 Non-TRKi Therapy
For patients receiving non-TRKi therapies at 1L (n = 98), median duration of 1L therapy was 5.6 months across all solid tumor types. Reasons for discontinuation of 1L non-TRKi therapies were due to disease progression (83.7%), completed scheduled duration of therapy (9.3%), patient choice (n = 2, 2.3%), toxicities/intolerability (n = 2, 2.3%), and death (n = 2, 2.3%). Median duration of non-TRKi therapies in the 2L setting (n = 30) was 5.3 months overall. Across combined hepatobiliary, as well as lung, cholangiocarcinoma, pancreatic, and colorectal cancers, the median duration of 2L non-TRKi therapy was 6.4, 5.6, 6.1, 7.7, and 4.6 months, respectively. Only 6 patients received non-TRKi therapies in the 3L setting, 2 with lung cancer, 3 with colorectal cancer, and 1 with thyroid cancer. Duration of non-TRKi therapy among the 6 patients was 4.9 months. Reasons for 2L and 3L non-TRKi discontinuation were largely due to disease progression (Table 4, Figs. 1, 2 and 3).
3.4.2 TRKi Therapy
Overall, patients who received TRKi therapy in the 1L setting (n = 12) showed longer 1L therapy duration at 16.8 months across all solid tumor types compared with those who did not receive TRKi therapy during 1L (median 1L duration of 5.6 months; p = 0.017). Among the solid tumor types represented by at least 10% of the study population, median duration of 1L TRKi therapy was only reached in patients with pancreatic cancer (3.3 months). Reasons for 1L TRKi therapy discontinuation were due to disease progression (n = 4, 80.0%) and completed scheduled duration of therapy (n = 1, 20.0%), but no patients died during 1L compared with 2 deaths during 1L among non-TRKi patients. Median duration of TRKi in the 2L setting (n = 48) was 7.9 months overall, relative to those who did not receive TRKi therapy during 2L (median 2L duration of 5.3 months; p = 0.003). Across combined hepatobiliary, as well as lung, cholangiocarcinoma, pancreatic, and colorectal cancers, the median duration of 2L TRKi therapy was 6.8, 14.1, 6.0, 6.1, and 4.1 months, respectively. There were 12 patients who received a TRKi therapy in the 3L setting; double the number receiving a non-TRKi therapy during 3L treatment (n = 6). In the 3L setting, median duration of TRKi therapy was 8.1 months among all patients (relative to 4.9 months among those who did not receive TRKi therapy during 3L; p = 0.005), 5.7 months among lung cancer patients, 6.1 months among cholangiocarcinoma patients, and not reached among pancreatic and colorectal cancer patients. Reasons for 2L and 3L TRKi discontinuation were largely due to disease progression, with death reported among 2 patients in 3L (Table 5, Figs. 1, 2 and 3).
4 Discussion
The present analysis represents the first real-world study of treatment patterns among patients with TRK fusion cancer.
When comparing the two treatment categories (TRKi vs non-TRKi), the median duration of 1L therapy was significantly shorter (5.6 months) among patients initiating a non-TRKi therapy during 1L, which was also the case in the 2L and 3L settings with median duration of 5.3 months and 4.9 months, respectively. Moreover, in the 3L setting, double the number of patients received TRKi therapies relative to non-TRKi therapies.
In the real-world setting, duration of 2L treatment reported by others for lung (2.9 months, 2L nivolumab [24]), pancreatic (1.9 months, 2L chemotherapy [25]), and colorectal cancer (4.5 months, 2L FOLFIRI-based regimens [26]) have ranged from 1.9 to 4.5 months. A previous study on a similar population describes timing of NTRK gene fusion testing and treatment modifications among patients with TRK fusion cancers [27]. In this study, where a majority of patients with TRK fusion cancer used TRKis in the 2L setting, the median duration of 2L TRKi therapy across lung, pancreatic, and colorectal cancers was 14.1, 6.1, and 4.1 months, respectively. While duration of treatment may provide real-world evidence of the effectiveness of a treatment, clinicians and other stakeholders may require assessment of real-world clinical outcomes to truly influence clinical practice, reimbursement policy, and formulary decisions.
Limitations of this study include the potential for patient and provider selection bias. It is possible that patient characteristics and clinical/treatment patterns may not be reflective of all patients within the TRK fusion solid tumor population. This study focused on adult patients, given the composition of the physician network (OPEN) used, despite TRK fusion cancer occurring in children and adolescents, albeit exceedingly rarely. Treatment with TRKi therapy may differ in younger populations, where entrectinib is approved for those aged ≥ 12 years and larotrectinib is approved for all ages. Moreover, the current study does not detail information about patients who initiate maintenance therapies. However, we have endeavored to attenuate such limitations to external validity by collecting data from oncologists in representative community practice settings. Second, certain data elements recorded by physicians in patient records may be recorded differently than those reported in clinical trials, as timepoints and criteria for assessment in clinical practice may be less stringent. Additionally, although duration of therapy is sometimes used as a surrogate for effectiveness, direct measures of effectiveness were not assessed in this study. Further research is warranted to assess these outcomes. Lastly, data recorded in patient medical records are intended to aid clinical management and not primarily for research purposes. Strengths of this study include its significant size (n = 110), given that TRK fusion cancer comprises only 1% of solid tumors, as well as duration of follow-up. Moreover, the selection of the cohort from community practices supports greater generalizability of the patient-level data on TRKi use in the real-world setting.
Future studies should evaluate clinical outcomes such as overall response rate, progression-free survival, and overall survival among patients with TRK fusion cancer treated with a TRKi compared to outcomes of those with TRK fusion cancer not treated with a TRKi. Furthermore, it remains unknown if earlier initiation and longer duration on TRKi therapy results in added clinical benefits when compared to TRKi initiation during 2L and subsequent lines of therapy. Additional research is needed to gain insight into the association between early TRKi therapy initiation and clinical outcomes such as progression-free survival and overall survival. Given the rarity of NTRK gene fusions in combination with tumor types such as cholangiocarcinoma and salivary gland, insights can be gleaned from the treatment patterns reported in this study.
5 Conclusion
The real-world evidence generated from this study provides valuable clinical insights for mitigating the unmet clinical needs of TRK fusion cancer by assessing the use of TRKis across different lines of therapy. Duration of therapy was significantly longer in both the 1L and 2L settings when treated with a TRKi therapy compared to other therapies among patients with a TRK fusion cancer. Further research is needed to assess clinical outcomes associated with early TRKi use, 1L versus late TRKi use (i.e., 2L and later lines) among patients with TRK fusion cancer for determining the most effective treatment strategies. As part of this study, participating physicians noted that, despite self-reported confidence in interpreting NTRK testing reports (96% reported little-to-no difficulty with interpretation), less than half (46%) included TRKi therapy following NTRK gene fusion determination. Thus, additional research is also needed to elucidate why some patients do not receive TRKi therapy after NTRK+ determination.
References
Davis JL, Lockwood CM, Albert CM, Tsuchiya K, Hawkins DS, Rudzinski ER. Infantile ntrk-associated mesenchymal tumors. Pediatr Dev Pathol. 2018;21:68–78.
Chmielecki J, Bailey M, He J, Elvin J, Vergilio JA, Ramkissoon S, et al. Genomic profiling of a large set of diverse pediatric cancers identifies known and novel mutations across tumor spectra. Cancer Res. 2017;77:509–19.
Orbach D, Brennan B, De Paoli A, Gallego S, Mudry P, Francotte N, et al. Conservative strategy in infantile fibrosarcoma is possible: the European paediatric soft tissue sarcoma study group experience. Eur J Cancer. 2016;57:1–9.
Rubin BP, Chen CJ, Morgan TW, Xiao S, Grier HE, Kozakewich HP, et al. Congenital mesoblastic nephroma t(12;15) is associated with etv6-ntrk3 gene fusion: cytogenetic and molecular relationship to congenital (infantile) fibrosarcoma. Am J Pathol. 1998;153:1451–8.
Bourgeois JM, Knezevich SR, Mathers JA, Sorensen PH. Molecular detection of the etv6-ntrk3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am J Surg Pathol. 2000;24:937–46.
Church AJ, Calicchio ML, Nardi V, Skalova A, Pinto A, Dillon DA, et al. Recurrent eml4-ntrk3 fusions in infantile fibrosarcoma and congenital mesoblastic nephroma suggest a revised testing strategy. Mod Pathol. 2018;31:463–73.
Horowitz DP, Sharma CS, Connolly E, Gidea-Addeo D, Deutsch I. Secretory carcinoma of the breast: Results from the survival, epidemiology and end results database. Breast. 2012;21:350–3.
Tognon C, Knezevich SR, Huntsman D, Roskelley CD, Melnyk N, Mathers JA, et al. Expression of the etv6-ntrk3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–76.
Bishop JA, Yonescu R, Batista D, Begum S, Eisele DW, Westra WH. Utility of mammaglobin immunohistochemistry as a proxy marker for the etv6-ntrk3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Hum Pathol. 2013;44:1982–8.
Skalova A, Vanecek T, Simpson RH, Laco J, Majewska H, Baneckova M, et al. Mammary analogue secretory carcinoma of salivary glands: Molecular analysis of 25 etv6 gene rearranged tumors with lack of detection of classical etv6-ntrk3 fusion transcript by standard rt-pcr: Report of 4 cases harboring etv6-x gene fusion. Am J Surg Pathol. 2016;40:3–13.
Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the etv6-ntrk3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608.
Penault-Llorca F, Rudzinski ER, Sepulveda AR. Testing algorithm for identification of patients with trk fusion cancer. J Clin Pathol. 2019;72:460–7.
Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46:444–50.
Demetri GD, Antonescu CR, Bjerkehagen B, Bovee J, Boye K, Chacon M, et al. Diagnosis and management of tropomyosin receptor kinase (trk) fusion sarcomas: expert recommendations from the world sarcoma network. Ann Oncol. 2020;31:1506–17.
Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, Yeh I, et al. Kinase fusions are frequent in spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116.
Yeh I, Tee MK, Botton T, Shain AH, Sparatta AJ, Gagnon A, et al. Ntrk3 kinase fusions in spitz tumours. J Pathol. 2016;240:282–90.
Lezcano C, Shoushtari AN, Ariyan C, Hollmann TJ, Busam KJ. Primary and metastatic melanoma with ntrk fusions. Am J Surg Pathol. 2018;42:1052–8.
Rosen EY, Goldman DA, Hechtman JF, Benayed R, Schram AM, Cocco E, et al. Trk fusions are enriched in cancers with uncommon histologies and the absence of canonical driver mutations. Clin Cancer Res. 2020;26:1624–32.
Okamura R, Boichard A, Kato S, Sicklick JK, Bazhenova L, Kurzrock R. Analysis of NTRK alterations in pan-cancer adult and pediatric malignancies: implications for NTRK-targeted therapeutics. JCO Precis Oncol. 2018;2018:PO.18.00183. https://doi.org/10.1200/PO.18.00183.
Solomon JP, Linkov I, Rosado A, Mullaney K, Rosen EY, Frosina D, et al. Ntrk fusion detection across multiple assays and 33,997 cases: diagnostic implications and pitfalls. Mod Pathol. 2020;33:38–46.
Solomon JP, Benayed R, Hechtman JF, Ladanyi M. Identifying patients with ntrk fusion cancer. Ann Oncol. 2019;30:viii16–22.
Food and Drug Administration. Fda approves larotrectinib for solid tumors with ntrk gene fusions. https://www.Fda.Gov/drugs/informationondrugs/approveddrugs/ucm626720.htm., 2018. Accessed 23 Aug 2021.
Food and Drug Administration. Fda approves entrectinib for ntrk solid tumors and ros-1 nsclc. https://www.Fda.Gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc. Accessed 23 Aug 2021.
Simeone JC, Nordstrom BL, Patel K, Klein AB. Treatment patterns and overall survival in metastatic non-small-cell lung cancer in a real-world, us setting. Future Oncol. 2019;15:3491–502.
Gransmark E, Bagenholm Bylin N, Blomstrand H, Fredrikson M, Avall-Lundqvist E, Elander NO. Real world evidence on second-line palliative chemotherapy in advanced pancreatic cancer. Front Oncol. 2020;10:1176.
Shinozaki E, Makiyama A, Kagawa Y, Satake H, Tanizawa Y, Cai Z, et al. Treatment sequences of patients with advanced colorectal cancer and use of second-line folfiri with antiangiogenic drugs in japan: a retrospective observational study using an administrative database. PLoS ONE. 2021;16: e0246160.
Klink AJ, Kavati A, Gassama AT, Kozlek T, Gajra A, Antoine R. Timing of ntrk gene fusion testing and treatment modifications following trk fusion status among us oncologists treating trk fusion cancer. Targeted Oncol. 2022;17:321–8.
Acknowledgments
We thank Danielle Gentile, Tammy Schuler, and Ryan Laughlin, all of Cardinal Health, for manuscript formatting assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Bayer.
Conflicts of interest/Competing interests
A.J.K. and A.G.: employees and owns stock in Cardinal Health. T.K.: employee and owns stock in Bayer U.S. A.K., R.A., T.A.G.: employees of Bayer U.S. at the time of study conduct.
Ethics approval and consent to participate
This research study was conducted retrospectively from data obtained for clinical purposes. As described in Methods, prior to chart data abstraction, study materials (research protocol and eCRF) were submitted to an independent, central Institutional Review Board (Western Institutional Review Board [IRB]). The IRB determined that this study met the criteria for a waiver of authorization for use and disclosure of PHI and was exempt from IRB oversight. A waiver of informed consent was granted.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are not publicly available due to presence of protected health information (PHI).
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by AJK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Klink, A.J., Kavati, A., Gassama, A. et al. Treatment Patterns of Real-World Patients with TRK Fusion Cancer Treated by US Community Oncologists. Targ Oncol 17, 549–561 (2022). https://doi.org/10.1007/s11523-022-00909-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00909-7