Abstract
Background
Neurotrophic tyrosine receptor kinase (NTRK) gene fusions are oncogenic drivers with an estimated prevalence of less than 1% across all solid tumors. Tropomyosin receptor kinase inhibitors (TRKis) block the constitutively activated tyrosine receptor kinase (TRK) fusion protein produced in NTRK gene fusion positive (NTRK+) tumors from downstream signaling. Tropomyosin receptor kinase inhibitors are now first-line (1L) or subsequent treatment options for TRK fusion cancers.
Objective
This study assessed timing of NTRK gene fusion testing and treatment modifications among patients with TRK fusion cancers.
Patients and Methods
This was a one-time physician questionnaire with a retrospective, multisite patient chart abstraction of oncology practices in the USA. From June to September 2020, medical oncologists from the Oncology Provider Extended Network (OPEN) who treated patients with NTRK+ advanced/metastatic solid tumors abstracted information into electronic case report forms (eCRFs) for adult patients with advanced/metastatic solid tumors and a NTRK+ tumor test result with a known fusion partner. Use of NTRK testing in routine clinical practice among patients with advanced/metastatic solid tumors was assessed. Data included demographic, clinical, and NTRK gene fusion testing characteristics. Responses were summarized using descriptive statistics.
Results
Twenty-eight community-based medical oncologists who had managed or treated 148 patients with advanced/metastatic TRK fusion cancer between 01/01/2016 and 12/31/2019 completed the survey. Lung (27%), thyroid (18%), salivary gland (14%), and colorectal (12%) were the most commonly reported tumor types. A majority (68%) tested NTRK status prior to 1L initiation; testing after disease progression on 1L (36%), 2L (25%), and 3L (21%) was also noted. Most oncologists (96%) reported no difficulty interpreting NTRK reports. Nearly all (96%) indicated using next-generation sequencing (NGS) for determining NTRK status. The majority (57%) indicated that age, tumor type, and performance status did not impact NTRK testing decisions. Less than half (46%) include TRKi therapy following NTRK+ determination. NTRK testing guidelines were commonly reviewed by physicians (89%).
Conclusion and Relevance
Among patients with advanced/metastatic TRK fusion cancer, medical oncologists reported testing for NTRK fusions at diagnosis or prior to 1L. Future research should elucidate why fewer than half of oncologists surveyed (46%) would not use TRKis after NTRK+ status confirmation, assess clinical practices among NTRK+ patients, and characterize treatment patterns and clinical outcomes in real-world settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Oncologists in this study reported that they use testing 68% of the time to identify cancers that have certain types of biomarkers, called tyrosine receptor kinase (TRK) fusions, prior to patients beginning systemic treatment such as chemotherapy. |
The majority of oncologists (96%) reported no difficulties interpreting TRK fusion cancer test reports and 89% indicated that they reviewed TRK fusion cancer testing guidelines regularly. |
However, less than half (46%) reported that they include tropomyosin receptor kinase inhibitors (TRKis), a targeted therapy for TRK fusion cancers, after a positive TRK fusion cancer test result. |
1 Introduction
Neurotrophic tyrosine receptor kinase (NTRK) gene fusions are oncogenic drivers that can present in any tumor type and may be implicated in approximately 1% of all solid tumor cancers [13]. The availability of tropomyosin receptor kinase inhibitors (TRKis) offers molecularly targeted therapy for patients with NTRK gene fusion positive (NTRK+) tumors [2, 11], but the low prevalence of such fusions and availability of testing modalities can be a potential barrier to their timely identification [9, 14].
Tropomyosin receptor kinase inhibitors target NTRK+ tumors by blocking the constitutively activated tyrosine receptor kinase (TRK) fusion protein produced by NTRK+ tumors from downstream signaling [14]. The US Food and Drug Administration (FDA) in 2018 approved larotrectinib and entrectinib for the treatment of TRK fusion cancer in both adult (larotrectinib and entrectinib: all ages) [4, 6, 10] and pediatric (larotrectinib: all ages, entrectinib: aged ≥ 12 years) populations [7]. Data that preceded the approval of larotrectinib indicated an overall response rate of 75%, including complete response among 13% and partial response among 62% of patients with NTRK+ tumors, irrespective of tumor type, patient age, or TRK fusion characteristics [5]. The National Comprehensive Cancer Network (NCCN) has not yet published guidelines for the use of tumor-agnostic treatments [1], however, to date, within the NCCN treatment guidelines, 25 different tumor types have incorporated treatment with a TRKi and/or testing for NTRK gene fusions.
A better understanding is need of how oncologists perform and assess genomic testing in the selection of systemic therapy for patients who may benefit from TRKi therapy. Such data will facilitate communication of both trial and real-world evidence (RWE) research results and could also be used to guide the dissemination of current and future TRKi therapy treatment guidelines. Finally, an understanding of the timing of NTRK gene fusion testing and overall physician perceptions of systemic therapy selection for TRK fusion cancers could help elucidate unmet needs among affected patients. Since TRKis are relatively new therapies and electronic medical records routinely fail to capture genomic testing accurately and uniquely, the existing evidence characterizing NTRK gene fusion testing patterns is therefore limited. This is particularly the case among data from patients treated outside of the clinical trial setting and outside of major cancer treatment centers.
Thus, the objective of this study was to evaluate real-world experiences of medical oncologists treating patients with TRK fusion cancers to assess timing of NTRK gene fusion testing and treatment modifications following an affirmation of NTRK+ tumor status.
2 Methods
Community-based medical oncologists from the Oncology Provider Extended Network (OPEN) in the USA, who had treated patients with TRK fusion cancer, were invited to participate in a retrospective patient chart review study. The OPEN community comprises more than 7000 unique providers in oncology, hematology, and urology across the USA. Over 800 of these physicians have participated in OPEN real-world research since 2016. Prior to chart data abstraction, study materials (research protocol and electronic case report forms [eCRF]) were submitted to an independent, central Institutional Review Board (Western Institutional Review Board [IRB]). The IRB determined that this study met the criteria for a waiver of authorization for use and disclosure of protected health information (PHI) and was exempt from IRB oversight. A waiver of informed consent was granted.
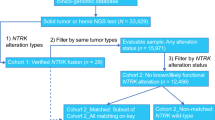
Medical oncologists from the OPEN community who had treated/managed adult patients with a TRK fusion cancer and had previously ordered NTRK gene fusion testing between January 1, 2016, and December 30, 2019, by any testing modality (e.g., next-generation sequencing [NGS], fluorescent in situ hybridization studies [FISH], immunohistochemistry [IHC]) were eligible for participation in the study. A one-time physician questionnaire collected oncologists’ experience with NTRK gene fusion testing in routine clinical practice among patients with advanced/metastatic solid tumors. This questionnaire of physicians was not directly related to the patient-level data, which was collected in a subsequent data abstraction of treatment patterns and outcomes for a separate publication.
Next, retrospective, de-identified patient-level data were abstracted by the participating medical oncologists. Each oncologist indicated solid tumor type for up to three patients with diagnoses of advanced/metastatic TRK fusion cancer. The limit of three eligible patients per provider, regardless of number of eligible patients with data available per provider, was imposed to minimize potential bias from a single practice. Oncologists also selected consecutively eligible patients to limit bias. Eligible patients were aged ≥ 18 years with a confirmed diagnosis of any advanced/metastatic solid tumor between January 1, 2016, and December 31, 2019, and had an NTRK+ tumor test result with a known fusion partner. Eligibility was limited to adult patients (aged ≥ 18 years) given the composition of the physician network. Patients were required to have at least three months of follow-up from the date of advanced/metastatic cancer diagnosis (unless deceased prior to 3 months following diagnosis). Patients were excluded with a prior primary cancer, diagnosed and treated within a year prior to 1L therapy initiation for the advanced/metastatic solid tumor of interest, as well as those participating in a clinical trial for treatment of their cancer. Data collection occurred from June through September 2020. Each completed eCRF was reviewed independently for implausible or inconsistent data. Providers were contacted with queries, and individual eCRFs that could not be validated were removed from the study dataset.
Responses regarding provider characteristics and NTRK gene fusion testing patterns from the physician questionnaire, as well as demographic, clinical characteristics, and treatment abstracted from patient medical records into eCRFs, were presented using descriptive statistics. All analyses were conducted in SAS v9.4.
3 Results
3.1 Provider Characteristics
In total, 28 medical oncologists provided data. Oncologists were geographically distributed across the USA: 25% Northeast, 14% Midwest, 39% South, 21% West. The majority (71%) reported their primary practice setting as private community practice, while 25% reported practicing at academic centers or affiliated teaching hospitals and 4% as solo practitioners. Urban, suburban, and rural practice settings were reported among 42.9%, 35.7%, and 21.4% of the medical oncologists, respectively. Oncologists’ median number of years in practice was 17 (range 13–23 years) and the oncologists had managed or treated 148 adult patients diagnosed with NTRK+ advanced/metastatic solid tumors between January 1, 2016, and December 31, 2019 (Table 1).
3.2 Neurotrophic Tyrosine Receptor Kinase Gene Fusion Testing and Treatment Decision Making
The majority of medical oncologists (57%) indicated that age, tumor type, and Eastern Cooperative Oncology Group (ECOG) performance status did not impact NTRK gene-fusion testing decisions (Fig. 1). When asked what training and/or resources were reviewed for NTRK gene fusion testing recommendations, medical oncologists reported that clinical guidelines (e.g., NCCN guidelines, ESMO Scale for Clinical Actionability of molecular Targets [ESCAT]) were most commonly reviewed (89%), followed by peer-reviewed literature (75%) and continuing medical education (57%) (Fig. 2). Over two-thirds (68%) of medical oncologists reported that they perform NTRK gene fusion testing at diagnosis (i.e., prior to 1L therapy initiation), over one-third perform testing following disease progression on 1L therapy (36%), one-quarter following 2L therapy (25%), and one-fifth following third-line (3L) therapy (21%) (Fig. 3). Nearly all (96%) indicated that they use NGS for determining NTRK gene fusion status, with FISH as the second most frequently used test (29%). Most medical oncologists (96%) self-reported little-to-no difficulty interpreting NTRK gene fusion testing reports. Of the medical oncologists self-reporting little-to-no difficulty interpreting reports, 39% indicated that they always have NTRK gene fusion testing reports interpreted by a third party, whereas 57% stated that they interpret the reports themselves. Treatment with a TRKi therapy following identification of a TRK fusion cancer was associated with fewer than half (46%) of responding medical oncologists. Of the 28 oncologists, 11% began testing for NTRK gene fusions in 2016 or 2017, 61% in 2018, and the remaining 28% in 2019.
Factors affecting medical oncologists’ decision-making process regarding NTRK gene fusion testing among patients with potentially NTRK+ tumors (listed are performance status, tumor type, patient age, or none of these). *Patient age, tumor type, ECOG-PS. ECOG-PS Eastern Cooperative Oncology Group Performance Status, NTRK+ neurotrophic tropomyosin-related kinase gene fusion-positive
Training and/or resources reviewed by medical oncologists for NTRK gene fusion testing recommendations (listed are published guidelines, peer-reviewed literature, continuing medical education, and institutional policies and updates). *e.g., ESMO, ESMO Scale for Clinical Actionability of Molecular Targets, National Comprehensive Cancer Network. ESMO European Society for Medical Oncology, NTRK+ neurotrophic tropomyosin-related kinase gene fusion
Timing of NTRK gene fusion testing (ranging from diagnosis to during later lines of therapy). Total percentage exceeds 100% as participating oncologists indicated timing of NTRK gene fusion testing across all NTRK+ patients. 1L first-line, 2L second-line, 3L third-line, NTRK+ neurotrophic tropomyosin-related kinase gene fusion-positive
3.3 Patient Demographics and Clinical Characteristics
The 28 participating medical oncologists entered patient-level data on 73 patients (of the 148 patients reported in total) with an NTRK+ solid tumor. Patient demographic and clinical characteristics are presented in Table 2. Patients’ median age at diagnosis of advanced/metastatic cancer was 61 years (range 55–68 years) and the overall sample showed a relatively even distribution between sex assigned at birth (52.1% male, 47.9% female). Lung (27.4%), thyroid (17.8%), salivary gland (13.7%) and colorectal (12.3%) were the most frequently reported tumor types, each present in > 10% of the cohort.
3.4 Testing Patterns
Among these 73 patients, NGS was used to identify NTRK gene fusions in 83.6% (including NGS large or full panel, NGS short or limited panel, and IHC followed by confirmatory NGS), followed by FISH in 15.1%. Testing for NTRK gene fusions occurred most frequently prior to 1L therapy initiation (43% of patients) and prior to second-line (2L) therapy initiation in 20.5% of patients. Larotrectinib (56%) and entrectinib (25%) were reported as the most commonly used treatments following identification of a TRK fusion cancer (Table 2).
4 Discussion
The present analysis represents the first real-world study of NTRK gene fusion testing patterns among oncologists. Study results demonstrated that medical oncologists most frequently reported testing for NTRK gene fusions at diagnosis, prior to the initiation of 1L therapy initiation. The majority (89%) reported having begun NTRK gene fusion testing in the post-approval era for larotrectinib [8] and entrectinib [7]. Oncologists also reported high confidence in their ability to interpret NTRK testing reports. As broad molecular profiling continues to evolve, correct interpretation and appropriate utilization of results will be critical to facilitate timely choice of cancer therapy. While NTRK gene fusions do exist across multiple tumor types and some are actionable for therapy selection, perception of actionable targets for tumor types may not be associated with genomic testing. A recent retrospective study found co-occurrence of NTRK gene fusions and other actionable biomarkers to be uncommon [3]
Despite self-reported physician confidence in interpreting NTRK testing reports, less than half (46%) reported including TRKi therapy following NTRK+ determination. The approval of larotrectinib3 was the second tumor‐agnostic FDA approval, following pembrolizumab, and the first approved TRKi for solid tumors that have an NTRK gene fusion. Currently, mismatch repair (MMR) and microsatellite instability (MSI) are the only other tumor agonistic biomarkers used to predict treatment response. Tyrosine receptor kinase fusion cancers are rare but additional research should elucidate why, despite NCCN recommendations across multiple tumor types, less than half (46%) of oncologists surveyed did not use TRKi therapy after NTRK+ confirmation.
Limitations of this study include the potential for patient and provider selection bias. For example, providers may come from cancer treatment centers with access to molecular pathologists to assist with NTRK testing report interpretations. Moreover, it is possible that patient characteristics and clinical/treatment patterns may not be reflective of all patients within the TRK fusion cancer population. This study focused on adult patients, given the composition of the physician network (OPEN) used, despite the higher incidence of TRK fusion cancers occurring in children and adolescents. Treatment with TRKi therapy may differ in younger populations, where entrectinib is approved for those aged ≥ 12 years and larotrectinib is approved for all ages. Moreover, the finding that most oncologists reported little-to-no difficulty in interpreting NTRK gene fusion testing reports may be associated with studying oncologists treating biomarker-driven tumors and may not apply to all providers. However, we have endeavored to attenuate such limitations to external validity by collecting data from oncologists in representative community practice settings. Second, certain data elements recorded by physicians in patient records may be recorded differently than those reported in clinical trials, as timepoints and criteria for assessment in clinical practice may be less stringent. Last, the primary purpose of data recorded in patient medical records are intended to aid clinical management and not research.
A strength of this study is its significant size since NTRK+ solid tumors comprise only 1% of solid tumors. Additionally, the required follow-up period for patients with abstracted chart data was at least three months. Finally, the selection of the cohort from community practices supports greater generalizability of the patient-level data on NTRK gene fusion testing and TRKi use in the real-world setting.
5 Conclusion
Among TRK fusion cancers, medical oncologists most frequently reported testing for NTRK gene fusions at diagnosis or prior to 1L therapy. Since less than one-third of oncologists report that ECOG performance status, tumor type, or patient age constitute factors that determine whether to test for NTRK gene fusions, further research is needed to identify physicians’ rationale for NTRK gene fusion testing. Despite nearly 90% of oncologists reporting that they review clinical guidelines to inform NTRK gene fusion testing, future research should also elucidate why less than half (46%) of oncologists surveyed would not use TRKi therapy after obtaining NTRK+ tumor confirmation. An opportunity to highlight treatment recommendations among patients with TRK fusion cancer in the commonly reviewed clinical guidelines (e.g., NCCN, European Society for Medical Oncology [ESMO], ESMO Scale for Clinical Actionability of molecular Targets [ESCAT]) exists to ensure that patients receive the most appropriate and timely treatment.
References
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Treatment by Cancer Type. https://www.nccn.org/guidelines/category_1. Accessed 19 July 2021.
Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016;1(2): e000023.
Bazhenova L, Lokker A, Snider J, Castellanos E, Fisher V, Fellous M, et al. TRK fusion cancer: patient characteristics and survival analysis in the real-world setting. Target Oncol. 2021;16(3):389–99.
Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):271–82.
Drilon A, Laetsch TW, Kummar S, Dubois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib inTRKFusion–positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9.
Filippi R, Depetris I, Satolli MA. Evaluating larotrectinib for the treatment of advanced solid tumors harboring an NTRK gene fusion. Expert Opin Pharmacother. 2021;22(6):677–84.
Food and Drug Administration FDA approves entrectinib for NTRK solid tumors and ROS-1 NSCLC. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc. Accessed 23 Aug 2021.
Food and Drug Administration. FDA approves larotrectinib for solid tumors with NTRK gene fusions. 2018. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm626720.htm. Accessed 23 Aug 2021.
Gatalica Z, Xiu J, Swensen J, Vranic S. Molecular characterization of cancers with NTRK gene fusions. Mod Pathol. 2019;32(1):147–53.
Hong DS, Dubois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531–40.
Khotskaya YB, Holla VR, Farago AF, Mills Shaw KR, Meric-Bernstam F, Hong DS. Targeting TRK family proteins in cancer. Pharmacol Ther. 2017;173:58–66.
Laetsch TW, Dubois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705–14.
Okamura R, Boichard A, Kato S, Sicklick JK, Bazhenova L, Kurzrock R (2018) Analysis of NTRK Alterations in Pan-Cancer Adult and Pediatric Malignancies: Implications for NTRK-Targeted Therapeutics. JCO Precis Oncol 2: 1-20
Solomon JP, Benayed R, Hechtman JF, Ladanyi M. Identifying patients with NTRK fusion cancer. Ann Oncol. 2019;30(Supplement_8):viii16–22.
Acknowledgments
We thank Danielle Gentile and Tammy Schuler, both of Cardinal Health, for manuscript formatting assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Bayer.
Conflicts of interest/competing interests:
A.J.K. and A.G.: employees and owns stock in Cardinal Health. T.K.: employee and owns stock in Bayer USA. A.K., R.A., T.A.G.: employees of Bayer USA at the time of study conduct.
Ethics approval and consent to participate
This research study was conducted retrospectively from data obtained for clinical purposes. As described in Methods, prior to chart data abstraction, study materials (research protocol and eCRF) were submitted to an independent, central Institutional Review Board (Western Institutional Review Board [IRB]). The IRB determined that this study met the criteria for a waiver of authorization for use and disclosure of PHI and was exempt from IRB oversight. A waiver of informed consent was granted.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are not publicly available due to presence of protected health information (PHI).
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by A.J.K. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Klink, A.J., Kavati, A., Gassama, A.T. et al. Timing of NTRK Gene Fusion Testing and Treatment Modifications Following TRK Fusion Status Among US Oncologists Treating TRK Fusion Cancer. Targ Oncol 17, 321–328 (2022). https://doi.org/10.1007/s11523-022-00887-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00887-w