Abstract
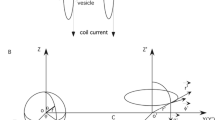
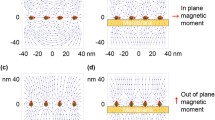
Cell membrane deforms in the electromagnetic field, suggesting an interesting control of cellular physiology by the field. Previous research has focused on the biomechanical analysis of membrane deformation under electric fields that are generated by electrodes. An alternative, noninvasive method to generate an electric field is the use of electromagnetic induction with a time-varying magnetic field, such as that used for transcranial magnetic stimulation (TMS). Although references reporting the magnetic control of cellular mechanics have recently emerged, theoretical analysis of the membrane biomechanics under a time-varying magnetic field is inadequate. We developed a cell model that included the membrane as a low-conductive, capacitive shell and investigated the electric pressure generated on the membrane by a low-frequency magnetic field (0–200 kHz). Our results show that externally applied magnetic field induced surface charges on both sides of the membrane. The charges interacted with the induced electric field to produce a radial pressure upon the membrane. Under the low-frequency range, the radial pressure pulled the cell membrane along the axis that was defined by the magnetically induced electric field. The radial pressure was a function of the field frequency, the conductivity ratio of the cytoplasm to the medium, and the size of the cell. It is quantitatively insignificant in deforming the membrane at the frequency used in TMS, but could be significant at a relatively higher-frequency range (>100 kHz).






Similar content being viewed by others
Abbreviations
- B o :
-
Intensity of the time-varying magnetic field (T)
- E :
-
Intensity of the electric field induced by time-varying magnetic field (V/m)
- ρ s01 :
-
Surface charge density on the medium/membrane interface (C/m2)
- ρ s12 :
-
Surface charge density on the membrane/cytoplasm interface (C/m2)
- Q s01 :
-
Net induced surface charges on the medium/membrane interface (C)
- Q s12 :
-
Net induced surface charges on the membrane/cytoplasm interface (C)
- P r01 :
-
Surface pressure on the medium/membrane interface (N/m2)
- P r12 :
-
Surface pressure on the membrane/cytoplasm interface (N/m2)
- P r :
-
Net surface pressure on the cell membrane (N/m2)
References
Anninos PA, Tsagas N, Sandyk R, Derpapas K (1991) Magnetic stimulation in the treatment of partial seizures. Int J Neurosci 60(3–4):141–171
Anninos PA, Tsagas N, Jacobson JI, Kotini A (1999) The biological effects of magnetic stimulation in epileptic patients. Panminerva Med 41(3):207–215
Aranda S, Riske KA, Lipowsky R, Dimova R (2008) Morphological transitions of vesicles induced by alternating electric fields. Biophys J 95(2):L19–L21. doi:10.1529/biophysj.108.132548
Barker AT, Garnham CW, Freeston IL (1991) Magnetic nerve stimulation: the effect of waveform on efficiency, determination of neural membrane time constants and the measurement of stimulator output. Electroencephalogr Clin Neurophysiol Suppl 43:227–237
Basser PJ, Roth BJ (1991) Stimulation of a myelinated nerve axon by electromagnetic induction. Med Biol Eng Comput 29(3):261–268
Bryant G, Wolfe J (1987) Electromechanical stresses produced in the plasma membranes of suspended cells by applied electric fields. J Membr Biol 96(2):129–139
Calvin NM, Hanawalt PC (1988) High-efficiency transformation of bacterial cells by electroporation. J Bacteriol 170(6):2796–2801
Darabi J, Guo C (2013) On-chip magnetophoretic isolation of CD4+ T cells from blood. Biomicrofluidics 7(5):54106. doi:10.1063/1.4821628
Dimova R, Riske KA, Aranda S, Bezlyepkina N, Knorr RL, Lipowsky R (2007) Giant vesicles in electric fields. Soft Matter 3(7):817–827. doi:10.1039/B703580b
Djamgoz MBA, Mycielska M, Madeja Z, Fraser SP, Korohoda W (2001) Directional movement of rat prostate cancer cells in direct-current electric field: involvement of voltage-gated Na+ channel activity. J Cell Sci 114(14):2697–2705
Engelhardt H, Sackmann E (1988) On the measurement of shear elastic moduli and viscosities of erythrocyte plasma membranes by transient deformation in high frequency electric fields. Biophys J 54(3):495–508. doi:10.1016/S0006-3495(88)82982-5
Epstein CM, Davey KR (2002) Iron-core coils for transcranial magnetic stimulation. J Clin Neurophysiol 19(4):376–381
Gabriel C, Gabriel S, Corthout E (1996) The dielectric properties of biological tissues: I. Literature survey. Phys Med Biol 41(11):2231–2249
Gehl J (2003) Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand 177(4):437–447
Gimsa J, Wachner D (2001) Analytical description of the transmembrane voltage induced on arbitrarily oriented ellipsoidal and cylindrical cells. Biophys J 81(4):1888–1896. doi:10.1016/S0006-3495(01)75840-7
Gimsa J, Wachner D (2001) On the analytical description of transmembrane voltage induced on spheroidal cells with zero membrane conductance. Eur Biophy J EBJ 30(6):463–466
Goldenberg NM, Steinberg BE (2010) Surface charge: a key determinant of protein localization and function. Cancer Res 70(4):1277–1280. doi:10.1158/0008-5472.CAN-09-2905
Griffiths DJ (1999) Introduction to electrodynamics, 3rd edn. Prentice-Hall Inc, New Jersey
Hyuga H, Kinosita K, Wakabayashi N (1991) Deformation of vesicles under the influence of strong electric-fields II. Jpn J Appl Phys 1 30(6):1333–1335. doi:10.1143/Jjap.30.1333
Jen DH, Steele CR (1987) Electrokinetic model of cochlear hair cell motility. J Acoust Soc Am 82(5):1667–1678
Kamm R, Lammerding J, Mofrad M (2010) Cellular nanomechanics. In: Bhushan B (ed) Springer handbook of nanotechnology, 3rd edn. Springer, Heidelberg, Dordrecht, London, New York, pp 1171–1200
Konings MK (2007) A new method for spatially selective, non-invasive activation of neurons: concept and computer simulation. Med Biol Eng Compu 45(1):7–24. doi:10.1007/s11517-006-0136-z
Kotnik T, Miklavcic D (2000) Analytical description of transmembrane voltage induced by electric fields on spheroidal cells. Biophys J 79(2):670–679. doi:10.1016/S0006-3495(00)76325-9
Kotnik T, Bobanovic F, Miklavcic D (1997) Sensitivity of transmembrane voltage induced by applied electric fields—a theoretical analysis. Bioelectrochem Bioenerg 43(2):285–291
Kotnik T, Bobanovic F, Miklavcic D (1997) Sensitivity of transmembrane voltage induced by applied electric fields—a theoretical analysis. Bioelectrochem Bioenerg 43(2):285–291. doi:10.1016/S0302-4598(97)00023-8
Kotnik T, Miklavcic D, Slivnik T (1998) Time course of transmembrane voltage induced by time-varying electric field—a method for theoretical analysis and its application. Bioelectrochem Bioenerg 45:3–16
Krasteva VT, Papazov SP, Daskalov IK (2003) Peripheral nerve magnetic stimulation: influence of tissue non-homogeneity. Biomed Eng Online 2:19. doi:10.1186/1475-925X-2-19
Lipman KM, Dodelson R, Hays RM (1966) The surface charge of isolated toad bladder epithelial cells. Mobility, effect of pH and divalent ions. J Gen Physiol 49(3):501–516
Mahaffy RE, Park S, Gerde E, Kas J, Shih CK (2004) Quantitative analysis of the viscoelastic properties of thin regions of fibroblasts using atomic force microscopy. Biophys J 86(3):1777–1793. doi:10.1016/S0006-3495(04)74245-9
McLaughlin S (1989) The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem 18:113–136. doi:10.1146/annurev.bb.18.060189.000553
Momen-Heravi F, Balaj L, Alian S, Mantel PY, Halleck AE, Trachtenberg AJ, Soria CE, Oquin S, Bonebreak CM, Saracoglu E, Skog J, Kuo WP (2013) Current methods for the isolation of extracellular vesicles. Biol Chem 394(10):1253–1262. doi:10.1515/hsz-2013-0141
Olivotto M, Arcangeli A, Carla M, Wanke E (1996) Electric fields at the plasma membrane level: a neglected element in the mechanisms of cell signalling. BioEssays 18(6):495–504. doi:10.1002/bies.950180612
Pasenkiewicz-Gierula M, Takaoka Y, Miyagawa H, Kitamura K, Kusumi A (1999) Charge pairing of headgroups in phosphatidylcholine membranes: a molecular dynamics simulation study. Biophys J 76(3):1228–1240. doi:10.1016/S0006-3495(99)77286-3
Polk C (1990) Electric fields and surface charges induced by ELF magnetic fields. Bioelectromagnetics 11(2):189–201
Polk C, Song JH (1990) Electric fields induced by low frequency magnetic fields in inhomogeneous biological structures that are surrounded by an electric insulator. Bioelectromagnetics 11(3):235–249
Polson MJ, Barker AT, Freeston IL (1982) Stimulation of nerve trunks with time-varying magnetic fields. Med Biol Eng Comput 20(2):243–244
Radmacher M, Cleveland JP, Fritz M, Hansma HG, Hansma PK (1994) Mapping interaction forces with the atomic force microscope. Biophys J 66(6):2159–2165. doi:10.1016/S0006-3495(94)81011-2
Reilly JP (1989) Peripheral nerve stimulation by induced electric currents: exposure to time-varying magnetic fields. Med Biol Eng Comput 27(2):101–110
Riske KA, Dimova R (2006) Electric pulses induce cylindrical deformations on giant vesicles in salt solutions. Biophys J 91(5):1778–1786. doi:10.1529/biophysj.106.081620
Rols MP, Delteil C, Serin G, Teissie J (1994) Temperature effects on electrotransfection of mammalian cells. Nucleic Acids Res 22(3):540
Roth BJ, Luterek A, Puwal S (2014) The movement of a nerve in a magnetic field: application to MRI Lorentz effect imaging. Med Biol Eng Comput 52(5):491–498. doi:10.1007/s11517-014-1153-y
Ruohonen J, Panizza M, Nilsson J, Ravazzani P, Grandori F, Tognola G (1996) Transverse-field activation mechanism in magnetic stimulation of peripheral nerves. Electroencephalogr Clin Neurophysiol 101(2):167–174
Sadik MM, Li J, Shan JW, Shreiber DI, Lin H (2011) Vesicle deformation and poration under strong dc electric fields. Phys Rev E Stat Nonlinear Soft Matter Phys 83(6 Pt 2):066316
Sandyk R, Anninos PA, Tsagas N, Derpapas K (1992) Magnetic fields in the treatment of Parkinson’s disease. Int J Neurosci 63(1–2):141–150
Schwan HP (1957) Electrical properties of tissue and cell suspensions. Adv Biol Med Phys 5:147–209
Sotiropoulos SN, Steinmetz PN (2007) Assessing the direct effects of deep brain stimulation using embedded axon models. J Neural Eng 4(2):107–119. doi:10.1088/1741-2560/4/2/011
Vlahovska PM, Gracia RS, Aranda-Espinoza S, Dimova R (2009) Electrohydrodynamic model of vesicle deformation in alternating electric fields. Biophys J 96(12):4789–4803. doi:10.1016/j.bpj.2009.03.054
Voldman J (2006) Electrical forces for microscale cell manipulation. Annu Rev Biomed Eng 8:425–454. doi:10.1146/annurev.bioeng.8.061505.095739
Wang N, Ingber DE (1994) Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J 66(6):2181–2189. doi:10.1016/S0006-3495(94)81014-8
Wang N, Butler JP, Ingber DE (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260(5111):1124–1127
Ye H, Curcuru A (2015) Vesicle biomechanics in a time-varying magnetic field. BMC Biophys 8(1):2. doi:10.1186/s13628-014-0016-0
Ye H, Steiger A (2015) Neuron matters: electric activation of neuronal tissue is dependent on the interaction between the neuron and the electric field. J Neuroeng Rehabil 12:65. doi:10.1186/s12984-015-0061-1
Ye H, Cotic M, Carlen PL (2007) Transmembrane potential induced in a spherical cell model under low-frequency magnetic stimulation. J Neural Eng 4(3):283–293. doi:10.1088/1741-2560/4/3/014
Ye H, Cotic M, Kang EE, Fehlings MG, Carlen PL (2010) Transmembrane potential induced on the internal organelle by a time-varying magnetic field: a model study. J Neuroeng Rehabil 7:12. doi:10.1186/1743-0003-7-12
Ye H, Cotic M, Fehlings MG, Carlen PL (2011) Transmembrane potential generated by a magnetically induced transverse electric field in a cylindrical axonal model. Med Biol Eng Comput 49(1):107–119. doi:10.1007/s11517-010-0704-0
Ye H, Cotic M, Fehlings MG, Carlen PL (2012) Influence of Cellular Properties on the Electric Field Distribution Around a Single Cell. Prog Electromagn Res B 39:141–161
Acknowledgments
The authors thank the Research Support Grant from Loyola University Chicago. Amanda Steiger assisted with the revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, H., Curcuru, A. Biomechanics of cell membrane under low-frequency time-varying magnetic field: a shell model. Med Biol Eng Comput 54, 1871–1881 (2016). https://doi.org/10.1007/s11517-016-1478-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-016-1478-9