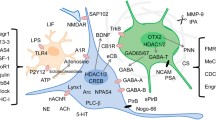
Abstract
BACKGROUND
Neuronal activity in cortical areas regulates neurodevelopment by interacting with defined genetic programs to shape the mature central nervous system. Electrical activity is conveyed to sensory cortical areas via intracortical and thalamocortical neurons, and includes oscillatory patterns that have been measured across cortical regions.
OBJECTIVE
In this work, we review the most recent findings about how electrical activity shapes the developmental assembly of functional circuitry in the somatosensory cortex, with an emphasis on interneuron maturation and integration. We include studies on the effect of various neurotransmitters and on the influence of thalamocortical afferent activity on circuit development. We additionally reviewed studies describing network activity patterns.
METHODS
We conducted an extensive literature search using both the PubMed and Google Scholar search engines. The following keywords were used in various iterations: “interneuron”, “somatosensory”, “development”, “activity”, “network patterns”, “thalamocortical”, “NMDA receptor”, “plasticity”. We additionally selected papers known to us from past reading, and those recommended to us by reviewers and members of our lab.
RESULTS
We reviewed a total of 132 articles that focused on the role of activity in interneuronal migration, maturation, and circuit development, as well as the source of electrical inputs and patterns of cortical activity in the somatosensory cortex. 79 of these papers included in this timely review were written between 2007 and 2016.
CONCLUSION
Neuronal activity shapes the developmental assembly of functional circuitry in the somatosensory cortical interneurons. This activity impacts nearly every aspect of development and acquisition of mature neuronal characteristics, and may contribute to changing phenotypes, altered transmitter expression, and plasticity in the adult. Progressively changing oscillatory network patterns contribute to this activity in the early postnatal period, although a direct requirement for specific patterns and origins of activity remains to be demonstrated.
Similar content being viewed by others
References
Adelsberger H, Garaschuk O, Konnerth A (2005). Cortical calcium waves in resting newborn mice. Nat Neurosci, 8(8): 988–990
Agmon A, Connors B W (1992). Correlation between intrinsic firing patterns and thalamocortical synaptic responses of neurons in mouse barrel cortex. J Neurosci, 12(1): 319–329
Agmon A, O’Dowd D K (1992). NMDA receptor-mediated currents are prominent in the thalamocortical synaptic response before maturation of inhibition. J Neurophysiol, 68(1): 345–349
Allène C, Cattani A, Ackman J B, Bonifazi P, Aniksztejn L, Ben-Ari Y, Cossart R (2008). Sequential generation of two distinct synapsedriven network patterns in developing neocortex. J Neurosci, 28(48): 12851–12863
Allene C, Cossart R (2010). Early NMDA receptor-driven waves of activity in the developing neocortex: physiological or pathological network oscillations? J Physiol, 588 (Pt 1): 83–91
An S, Kilb W, Luhmann H J (2014). Sensory-evoked and spontaneous gamma and spindle bursts in neonatal rat motor cortex. J Neurosci, 34(33): 10870–10883
Anastasiades P G, Marques-Smith A, Lyngholm D, Lickiss T, Raffiq S, Kätzel D, Miesenböck G, Butt S J (2016). GABAergic interneurons form transient layer-specific circuits in early postnatal neocortex. Nat Commun, 7: 10584
Arroyo D A, Feller M B (2016). Spatiotemporal Features of Retinal Waves Instruct the Wiring of the Visual Circuitry. Front Neural Circuits, 10: 54
Ascoli G A, Alonso-Nanclares L, Anderson S A, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsáki G, Cauli B, Defelipe J, Fairén A, Feldmeyer D, Fishell G, Fregnac Y, Freund T F, Gardner D, Gardner E P, Goldberg J H, Helmstaedter M, Hestrin S, Karube F, Kisvárday Z F, Lambolez B, Lewis D A, Marin O, Markram H, Muñoz A, Packer A, Petersen C C, Rockland K S, Rossier J, Rudy B, Somogyi P, Staiger J F, Tamas G, Thomson A M, Toledo-Rodriguez M, Wang Y, West D C, Yuste R, Yuste R, and the Petilla Interneuron Nomenclature Group (2008). Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci, 9(7): 557–568
Avila A, Vidal P M, Dear T N, Harvey R J, Rigo J M, Nguyen L (2013). Glycine receptor a2 subunit activation promotes cortical interneuron migration. Cell Reports, 4(4): 738–750
Baho E, Di Cristo G (2012). Neural activity and neurotransmission regulate the maturation of the innervation field of cortical GABAergic interneurons in an age-dependent manner. J Neurosci, 32(3): 911–918
Bando Y, Irie K, Shimomura T, Umeshima H, Kushida Y, Kengaku M, Fujiyoshi Y, Hirano T, Tagawa Y (2016). Control of spontaneous Ca2+ transients is critical for neuronal maturation in the developing neocortex. Cereb Cortex, 26(1): 106–117
Batista-Brito R, Fishell G (2009). The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol, 87: 81–118
Batista-Brito R, Machold R, Klein C, Fishell G (2008). Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb Cortex, 18(10): 2306–2317
Behar T N, Schaffner A E, Scott C A, Greene C L, Barker J L (2000). GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex, 10(9): 899–909
Behar T N, Scott C A, Greene C L, Wen X, Smith S V, Maric D, Liu Q Y, Colton C A, Barker J L (1999). Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration. J Neurosci, 19(11): 4449–4461
Bony G, Szczurkowska J, Tamagno I, Shelly M, Contestabile A, Cancedda L (2013). Non-hyperpolarizing GABAB receptor activation regulates neuronal migration and neurite growth and specification by cAMP/LKB1. Nat Commun, 4: 1800
Bortone D, Polleux F (2009). KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron, 62(1): 53–71
Butt S J, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin J G, Fishell G (2005). The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron, 48(4): 591–604
Campanac E, Gasselin C, Baude A, Rama S, Ankri N, Debanne D (2013). Enhanced intrinsic excitability in basket cells maintains excitatory-inhibitory balance in hippocampal circuits. Neuron, 77(4): 712–722
Chaudhury S, Sharma V, Kumar V, Nag T C, Wadhwa S (2016). Activity-dependent synaptic plasticity modulates the critical phase of brain development. Brain Dev, 38(4): 355–363
Chu J, Anderson S A (2015). Development of cortical interneurons. Neuropsychopharmacology, 40(1): 16–23
Close J, Xu H, De Marco García N, Batista-Brito R, Rossignol E, Rudy B, Fishell G (2012). Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. J Neurosci, 32(49): 17690–17705
Cohen-Kashi Malina K, Mohar B, Rappaport A N, Lampl I (2016). Local and thalamic origins of correlated ongoing and sensory-evoked cortical activities. Nat Commun, 7: 12740
Colonnese M T, Kaminska A, Minlebaev M, Milh M, Bloem B, Lescure S, Moriette G, Chiron C, Ben-Ari Y, Khazipov R (2010). A conserved switch in sensory processing prepares developing neocortex for vision. Neuron, 67(3): 480–498
Conhaim J, Easton C R, Becker M I, Barahimi M, Cedarbaum E R, Moore J G, Mather L F, Dabagh S, Minter D J, Moen S P, Moody W J (2011). Developmental changes in propagation patterns and transmitter dependence of waves of spontaneous activity in the mouse cerebral cortex. J Physiol, 589 (Pt 10): 2529–2541
Corlew R, Bosma M M, Moody W J (2004). Spontaneous, synchronous electrical activity in neonatal mouse cortical neurones. J Physiol, 560 (Pt 2): 377–390
Cossart R, Ikegaya Y, Yuste R (2005). Calcium imaging of cortical networks dynamics. Cell Calcium, 37(5): 451–457
Coulter D A (2001). Epilepsy-associated plasticity in gamma-aminobutyric acid receptor expression, function, and inhibitory synaptic properties. Int Rev Neurobiol, 45: 237–252
Crair M C, Malenka R C (1995). A critical period for long-term potentiation at thalamocortical synapses. Nature, 375(6529): 325–328
Cruikshank S J, Urabe H, Nurmikko A V, Connors B W (2010). Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron, 65(2): 230–245
Cuzon V C, Yeh P W, Cheng Q, Yeh H H (2006). Ambient GABA promotes cortical entry of tangentially migrating cells derived from the medial ganglionic eminence. Cereb Cortex, 16(10): 1377–1388
Cuzon Carlson V C, Yeh H H (2011). GABAA receptor subunit profiles of tangentially migrating neurons derived from the medial ganglionic eminence. Cereb Cortex, 21(8): 1792–1802
Daw M I, Scott H L, Isaac J T (2007). Developmental synaptic plasticity at the thalamocortical input to barrel cortex: mechanisms and roles. Mol Cell Neurosci, 34(4): 493–502
de Lima A D, Gieseler A, Voigt T (2009). Relationship between GABAergic interneurons migration and early neocortical network activity. Dev Neurobiol, 69 (2-3): 105–123
De Marco García N V, Karayannis T, Fishell G (2011). Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature, 472(7343): 351–355
De Marco García N V, Priya R, Tuncdemir S N, Fishell G, Karayannis T (2015). Sensory inputs control the integration of neurogliaform interneurons into cortical circuits. Nat Neurosci, 18(3): 393–401
De Diego I, Smith-Fernández A, Fairén A (1994). Cortical cells that migrate beyond area boundaries: characterization of an early neuronal population in the lower intermediate zone of prenatal rats. Eur J Neurosci, 6(6): 983–997
Dehorter N, Ciceri G, Bartolini G, Lim L, Del Pino I, Marín O (2015). Tuning of fast-spiking interneuron properties by an activitydependent transcriptional switch. Science, 349(6253): 1216–1220
Denaxa M, Kalaitzidou M, Garefalaki A, Achimastou A, Lasrado R, Maes T, Pachnis V (2012). Maturation-promoting activity of SATB1 in MGE-derived cortical interneurons. Cell Reports, 2(5): 1351–1362
Dupont E, Hanganu I L, Kilb W, Hirsch S, Luhmann H J (2006). Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature, 439(7072): 79–83
Easton C R, Weir K, Scott A, Moen S P, Barger Z, Folch A, Hevner R F, Moody W J (2014). Genetic elimination of GABAergic neurotransmission reveals two distinct pacemakers for spontaneous waves of activity in the developing mouse cortex. J Neurosci, 34(11): 3854–3863
Erzurumlu R S, Gaspar P (2012). Development and critical period plasticity of the barrel cortex. Eur J Neurosci, 35(10): 1540–1553
Espinosa J S, Stryker M P (2012). Development and plasticity of the primary visual cortex. Neuron, 75(2): 230–249
Espinosa J S, Wheeler D G, Tsien R W, Luo L (2009). Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron, 62(2): 205–217
Feldmeyer D (2012). Excitatory neuronal connectivity in the barrel cortex. Front Neuroanat, 6: 24
Feldmeyer D, Brecht M, Helmchen F, Petersen C C, Poulet J F, Staiger J F, Luhmann H J, Schwarz C (2013). Barrel cortex function. Prog Neurobiol, 103: 3–27
Fishell G, Rudy B (2011). Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu Rev Neurosci, 34(1): 535–567
Flint A C, Maisch U S, Weishaupt J H, Kriegstein A R, Monyer H (1997). NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci, 17(7): 2469–2476
Frazer S, Otomo K, Dayer A (2015). Early-life serotonin dysregulation affects the migration and positioning of cortical interneuron subtypes. Transl Psychiatry, 5 (9): e644
Garaschuk O, Linn J, Eilers J, Konnerth A (2000). Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci, 3(5): 452–459
Gierdalski M, Jablonska B, Siucinska E, Lech M, Skibinska A, Kossut M (2001). Rapid regulation of GAD67 mRNA and protein level in cortical neurons after sensory learning. Cereb Cortex, 11(9): 806–815
Golshani P, Gonçalves J T, Khoshkhoo S, Mostany R, Smirnakis S, Portera-Cailliau C (2009). Internally mediated developmental desynchronization of neocortical network activity. J Neurosci, 29(35): 10890–10899
Hanganu I L, Kilb W, Luhmann H J (2002). Functional synaptic projections onto subplate neurons in neonatal rat somatosensory cortex. J Neurosci, 22(16): 7165–7176
Heck N, Kilb W, Reiprich P, Kubota H, Furukawa T, Fukuda A, Luhmann H J (2007). GABA-A receptors regulate neocortical neuronal migration in vitro and in vivo. Cereb Cortex, 17(1): 138–148
Higashi S, Hioki K, Kurotani T, Kasim N, Molnár Z (2005). Functional thalamocortical synapse reorganization from subplate to layer IV during postnatal development in the reeler-like mutant rat (shaking rat Kawasaki). J Neurosci, 25(6): 1395–1406
Huang Z J, Di Cristo G, Ango F (2007). Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci, 8(9): 673–686
Inada H, Watanabe M, Uchida T, Ishibashi H, Wake H, Nemoto T, Yanagawa Y, Fukuda A, Nabekura J (2011). GABA regulates the multidirectional tangential migration of GABAergic interneurons in living neonatal mice. PLoS ONE, 6 (12): e27048
Iwasato T, Datwani A, Wolf A M, Nishiyama H, Taguchi Y, Tonegawa S, Knöpfel T, Erzurumlu R S, Itohara S (2000). Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature, 406(6797): 726–731
Ji X Y, Zingg B, Mesik L, Xiao Z, Zhang L I, Tao H W (2016). Thalamocortical Innervation Pattern in Mouse Auditory and Visual Cortex: Laminar and Cell-Type Specificity. Cereb Cortex, 26(6): 2612–2625
Jiao Y, Zhang C, Yanagawa Y, Sun Q Q (2006). Major effects of sensory experiences on the neocortical inhibitory circuits. J Neurosci, 26(34): 8691–8701
Kanold P O (2004). Transient microcircuits formed by subplate neurons and their role in functional development of thalamocortical connections. Neuroreport, 15(14): 2149–2153
Kanold P O, Kara P, Reid R C, Shatz C J (2003). Role of subplate neurons in functional maturation of visual cortical columns. Science, 301(5632): 521–525
Kanold P O, Luhmann H J (2010). The subplate and early cortical circuits. Annu Rev Neurosci, 33(1): 23–48
Karayannis T, De Marco García N V, Fishell G J (2012). Functional adaptation of cortical interneurons to attenuated activity is subtypespecific. Front Neural Circuits, 6: 66
Karnani M M, Jackson J, Ayzenshtat I, Tucciarone J, Manoocheri K, Snider W G, Yuste R (2016). Cooperative Subnetworks of Molecularly Similar Interneurons in Mouse Neocortex. Neuron, 90(1): 86–100
Kepecs A, Fishell G (2014). Interneuron cell types are fit to function. Nature, 505(7483): 318–326
Khazipov R, Luhmann H J (2006). Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci, 29(7): 414–418
Khazipov R, Sirota A, Leinekugel X, Holmes G L, Ben-Ari Y, Buzsáki G (2004). Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature, 432(7018): 758–761
Kihara M, Yoshioka H, Hirai K, Hasegawa K, Kizaki Z, Sawada T (2002). Stimulation of N-methyl-D-aspartate (NMDA) receptors inhibits neuronal migration in embryonic cerebral cortex: a tissue culture study. Brain Res Dev Brain Res, 138(2): 195–198
Kilb W, Kirischuk S, Luhmann H J (2011). Electrical activity patterns and the functional maturation of the neocortex. Eur J Neurosci, 34(10): 1677–1686
Kilb W, Kirischuk S, Luhmann H J (2013). Role of tonic GABAergic currents during pre- and early postnatal rodent development. Front Neural Circuits, 7: 139
Killackey H P (1973). Anatomical evidence for cortical subdivisions based on vertically discrete thalamic projections from the ventral posterior nucleus to cortical barrels in the rat. Brain Res, 51: 326–331
Kirmse K, Kummer M, Kovalchuk Y, Witte O W, Garaschuk O, Holthoff K (2015). GABA depolarizes immature neurons and inhibits network activity in the neonatal neocortex in vivo. Nat Commun, 6: 7750
Koolen N, Dereymaeker A, Räsänen O, Jansen K, Vervisch J, Matic V, Naulaers G, de Vos M, Van Huffel S, Vanhatalo S (2016). Early development of synchrony in cortical activations in the human. Neuroscience, 322: 298–307
Kral A (2013). Auditory critical periods: a review from system’s perspective. Neuroscience, 247: 117–133
Laaris N, Carlson G C, Keller A (2000). Thalamic-evoked synaptic interactions in barrel cortex revealed by optical imaging. J Neurosci, 20(4): 1529–1537
Lee L J, Iwasato T, Itohara S, Erzurumlu R S (2005). Exuberant thalamocortical axon arborization in cortex-specific NMDAR1 knockout mice. J Comp Neurol, 485(4): 280–292
Lewis D A (2014). Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol, 26: 22–26
Li H, Fertuzinhos S, Mohns E, Hnasko T S, Verhage M, Edwards R, Sestan N, Crair M C (2013). Laminar and columnar development of barrel cortex relies on thalamocortical neurotransmission. Neuron, 79(5): 970–986
Liang F, Isackson P J, Jones E G (1996). Stimulus-dependent, reciprocal up- and downregulation of glutamic acid decarboxylase and Ca2+/ calmodulin-dependent protein kinase II gene expression in rat cerebral cortex. Exp Brain Res, 110(2): 163–174
Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V (2007). Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci, 27(12): 3078–3089
Liu X, Hashimoto-Torii K, Torii M, Ding C, Rakic P (2010). Gap junctions/hemichannels modulate interkinetic nuclear migration in the forebrain precursors. J Neurosci, 30(12): 4197–4209
Liu X, Hashimoto-Torii K, Torii M, Haydar T F, Rakic P (2008). The role of ATP signaling in the migration of intermediate neuronal progenitors to the neocortical subventricular zone. Proc Natl Acad Sci USA, 105(33): 11802–11807
Liu X B, Murray K D, Jones E G (2004). Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci, 24(40): 8885–8895
Lorente de No R (1922). La Corteza Cerebral del Raton (Primera Contribucion- La Corteza Acustica). Trabajos del Laboratorio de Investigaciones Biologicas, 20: 41–78
Lo Turco J J, Blanton M G, Kriegstein A R (1991). Initial expression and endogenous activation of NMDA channels in early neocortical development. J Neurosci, 11(3): 792–799
Luhmann H J, Fukuda A, Kilb W (2015). Control of cortical neuronal migration by glutamate and GABA. Front Cell Neurosci, 9: 4
Luhmann H J, Hanganu I, Kilb W (2003). Cellular physiology of the neonatal rat cerebral cortex. Brain Res Bull, 60(4): 345–353
Luhmann H J, Kirischuk S, Sinning A, Kilb W (2014). Early GABAergic circuitry in the cerebral cortex. Curr Opin Neurobiol, 26: 72–78
Manent J B, Jorquera I, Ben-Ari Y, Aniksztejn L, Represa A (2006). Glutamate acting on AMPA but not NMDA receptors modulates the migration of hippocampal interneurons. J Neurosci, 26(22): 5901–5909
Marín O (2012). Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci, 13(2): 107–120
Marques-Smith A, Lyngholm D, Kaufmann A K, Stacey J A, Hoerder-Suabedissen A, Becker E B, Wilson M C, Molnár Z, Butt S J (2016). A Transient Translaminar GABAergic Interneuron Circuit Connects Thalamocortical Recipient Layers in Neonatal Somatosensory Cortex. Neuron, 89(3): 536–549
Matta J A, Pelkey K A, Craig M T, Chittajallu R, Jeffries B W, McBain C J (2013). Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci, 16(8): 1032–1041
McCabe A K, Chisholm S L, Picken-Bahrey H L, Moody W J (2006). The self-regulating nature of spontaneous synchronized activity in developing mouse cortical neurones. J Physiol, 577 (Pt 1): 155–167
Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, Khazipov R (2007). Rapid cortical oscillations and early motor activity in premature human neonate. Cereb Cortex, 17(7): 1582–1594
Minlebaev M, Ben-Ari Y, Khazipov R (2007). Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. J Neurophysiol, 97(1): 692–700
Minlebaev M, Ben-Ari Y, Khazipov R (2009). NMDA receptors pattern early activity in the developing barrel cortex in vivo. Cereb Cortex, 19(3): 688–696
Minlebaev M, Colonnese M, Tsintsadze T, Sirota A, Khazipov R (2011). Early g oscillations synchronize developing thalamus and cortex. Science, 334(6053): 226–229
Mix A, Hoppenrath K, Funke K (2015). Reduction in cortical parvalbumin expression due to intermittent theta-burst stimulation correlates with maturation of the perineuronal nets in young rats. Dev Neurobiol, 75(1): 1–11
Miyashita-Lin EM, Hevner R, Wassarman KM, Martinez S, Rubenstein J L (1999). Early neocortical regionalization in the absence of thalamic innervation. Science, 285(5429): 906–909
Miyoshi G, Butt S J, Takebayashi H, Fishell G (2007). Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci, 27(29): 7786–7798
Miyoshi G, Fishell G (2011). GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex, 21(4): 845–852
Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa V H, Butt S J, Battiste J, Johnson J E, Machold R P, Fishell G (2010). Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci, 30(5): 1582–1594
Mizuno H, Luo W, Tarusawa E, Saito Y M, Sato T, Yoshimura Y, Itohara S, Iwasato T (2014). NMDAR-regulated dynamics of layer 4 neuronal dendrites during thalamocortical reorganization in neonates. Neuron, 82(2): 365–379
Molnár Z, Adams R, Blakemore C (1998). Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci, 18(15): 5723–5745
Murthy S, Niquille M, Hurni N, Limoni G, Frazer S, Chameau P, Van Hooft J A, Vitalis T, Dayer A (2014). Serotonin receptor 3A controls interneuron migration into the neocortex. Nat Commun, 5: 5524
Narboux-Nême N, Evrard A, Ferezou I, Erzurumlu R S, Kaeser P S, Lainé J, Rossier J, Ropert N, Südhof T C, Gaspar P (2012). Neurotransmitter release at the thalamocortical synapse instructs barrel formation but not axon patterning in the somatosensory cortex. J Neurosci, 32(18): 6183–6196
Oh W C, Lutzu S, Castillo P E, Kwon H B (2016). De novo synaptogenesis induced by GABA in the developing mouse cortex. Science, 353(6303): 1037–1040
Okaty B W, Miller M N, Sugino K, Hempel C M, Nelson S B (2009). Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci, 29(21): 7040–7052
Paoletti P, Bellone C, Zhou Q (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci, 14(6): 383–400
Petersen C C (2007). The functional organization of the barrel cortex. Neuron, 56(2): 339–355
Porter J T, Johnson C K, Agmon A (2001). Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci, 21(8): 2699–2710
Reiprich P, Kilb W, Luhmann H J (2005). Neonatal NMDA receptor blockade disturbs neuronal migration in rat somatosensory cortex in vivo. Cereb Cortex, 15(3): 349–358
Rheims S, Minlebaev M, Ivanov A, Represa A, Khazipov R, Holmes G L, Ben-Ari Y, Zilberter Y (2008). Excitatory GABA in rodent developing neocortex in vitro. J Neurophysiol, 100(2): 609–619
Riccio O, Potter G, Walzer C, Vallet P, Szabó G, Vutskits L, Kiss J Z, Dayer A G (2009). Excess of serotonin affects embryonic interneuron migration through activation of the serotonin receptor 6. Mol Psychiatry, 14(3): 280–290
Rudy B, Fishell G, Lee S, Hjerling-Leffler J (2011). Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol, 71(1): 45–61
Rutherford L C, De Wan A, Lauer H M, Turrigiano G G (1997). Brainderived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci, 17(12): 4527–4535
Sanes D H, Kotak V C (2011). Developmental plasticity of auditory cortical inhibitory synapses. Hear Res, 279 (1-2): 140–148
Schwartz T H, Rabinowitz D, Unni V, Kumar V S, Smetters D K, Tsiola A, Yuste R (1998). Networks of coactive neurons in developing layer 1. Neuron, 20(3): 541–552
Siegel F, Heimel J A, Peters J, Lohmann C (2012). Peripheral and central inputs shape network dynamics in the developing visual cortex in vivo. Curr Biol, 22(3): 253–258
Sippy T, Yuste R (2013). Decorrelating action of inhibition in neocortical networks. J Neurosci, 33(23): 9813–9830
Soria J M, Valdeolmillos M (2002). Receptor-activated calcium signals in tangentially migrating cortical cells. Cereb Cortex, 12(8): 831–839
Stosiek C, Garaschuk O, Holthoff K, Konnerth A (2003). In vivo twophoton calcium imaging of neuronal networks. Proc Natl Acad Sci USA, 100(12): 7319–7324
Sultan K T, Brown K N, Shi S H (2013). Production and organization of neocortical interneurons. Front Cell Neurosci, 7: 221
Sun J J, Luhmann H J (2007). Spatio-temporal dynamics of oscillatory network activity in the neonatal mouse cerebral cortex. Eur J Neurosci, 26(7): 1995–2004
Sun Q Q, Huguenard J R, Prince D A (2006). Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci, 26(4): 1219–1230
Sur M, Leamey C A (2001). Development and plasticity of cortical areas and networks. Nat Rev Neurosci, 2(4): 251–262
Takano T (2015). Interneuron dysfunction in syndromic autism: Recent advances. Dev Neurosci, 37(6): 467–475
Tasic B, Menon V, Nguyen T N, Kim T K, Jarsky T, Yao Z, Levi B, Gray L T, Sorensen S A, Dolbeare T, Bertagnolli D, Goldy J, Shapovalova N, Parry S, Lee C, Smith K, Bernard A, Madisen L, Sunkin S M, Hawrylycz M, Koch C, Zeng H (2016). Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci, 19(2): 335–346
Tolner E A, Sheikh A, Yukin A Y, Kaila K, Kanold P O (2012). Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. J Neurosci, 32(2): 692–702
Tolonen M, Palva J M, Andersson S, Vanhatalo S (2007). Development of the spontaneous activity transients and ongoing cortical activity in human preterm babies. Neuroscience, 145(3): 997–1006
Trevelyan A J, Muldoon S F, Merricks E M, Racca C, Staley K J (2015). The role of inhibition in epileptic networks. J Clin Neurophysiol, 32(3): 227–234
Tuncdemir S N, Wamsley B, Stam F J, Osakada F, Goulding M, Callaway E M, Rudy B, Fishell G (2016). Early Somatostatin Interneuron Connectivity Mediates the Maturation of Deep Layer Cortical Circuits. Neuron, 89(3): 521–535
Uhlén P, Fritz N, Smedler E, Malmersjö S, Kanatani S (2015). Calcium signaling in neocortical development. Dev Neurobiol, 75(4): 360–368
van der Loos H, Woolsey T A (1973). Somatosensory cortex: structural alterations following early injury to sense organs. Science, 179(4071): 395–398
Van Eden C G, Mrzljak L, Voorn P, Uylings H B (1989). Prenatal development of GABA-ergic neurons in the neocortex of the rat. J Comp Neurol, 289(2): 213–227
Vitalis T, Ansorge M S, Dayer A G (2013). Serotonin homeostasis and serotonin receptors as actors of cortical construction: special attention to the 5-HT3A and 5-HT6 receptor subtypes. Front Cell Neurosci, 7: 93
Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas J G (2007). Embryonic depletion of serotonin affects cortical development. Eur J Neurosci, 26(2): 331–344
Voigt T, Opitz T, de Lima A D (2001). Synchronous oscillatory activity in immature cortical network is driven by GABAergic preplate neurons. J Neurosci, 21(22): 8895–8905
Welker C (1971). Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res, 26(2): 259–275
Welker C (1976). Receptive fields of barrels in the somatosensory neocortex of the rat. J Comp Neurol, 166(2): 173–189
White L E, Fitzpatrick D (2007). Vision and cortical map development. Neuron, 56(2): 327–338
Wichterle H, Garcia-Verdugo J M, Herrera D G, Alvarez-Buylla A (1999). Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci, 2(5): 461–466
Wichterle H, Turnbull D H, Nery S, Fishell G, Alvarez-Buylla A (2001). In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development, 128(19): 3759–3771
Woolsey T A, van der Loos H (1970). The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res, 17(2): 205–242
Wu X, Fu Y, Knott G, Lu J, Di Cristo G, Huang Z J (2012). GABA signaling promotes synapse elimination and axon pruning in developing cortical inhibitory interneurons. J Neurosci, 32(1): 331–343
Yang J W, Hanganu-Opatz I L, Sun J J, Luhmann H J (2009). Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J Neurosci, 29(28): 9011–9025
Yang JW, Reyes-Puerta V, Kilb W, Luhmann H J (2016). Spindle Bursts in Neonatal Rat Cerebral Cortex. Neural Plast, 2016: 3467832
Yozu M, Tabata H, Konig N, Nakajima K (2008). Migratory behavior of presumptive interneurons is affected by AMPA receptor activation in slice cultures of embryonic mouse neocortex. Dev Neurosci, 30 (1-3): 105–116
Zeisel A, Muñoz-Manchado A B, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo-Branco G, Hjerling-Leffler J, Linnarsson S (2015). Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science, 347(6226): 1138–1142
Zhang Z, Sun Q Q (2011). Development of NMDA NR2 subunits and their roles in critical period maturation of neocortical GABAergic interneurons. Dev Neurobiol, 71(3): 221–245
Acknowledgments
We are grateful to A. Che, P. Chu, and S. Z. Duan for comments on the manuscript. N.V.D.M.G. is supported by grants from the National Institutes of Health (5 R00 MH095825 05; 1 R01 MH110553 01), the Leon Levy Foundation, and Citizens United for Research in Epilepsy (CURE). R.B. is supported by a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the NIH under award number T32GM07739 to the Weill Cornell/Rockefeller/ Sloan-Kettering Tri-Institutional MD-PhD Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babij, R., De Marco Garcia, N. Neuronal activity controls the development of interneurons in the somatosensory cortex. Front. Biol. 11, 459–470 (2016). https://doi.org/10.1007/s11515-016-1427-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-016-1427-x