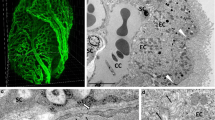
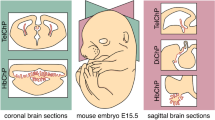
Abstract
The choroid plexus (CP), localized in brain ventricles, is the major source of cerebrospinal fluid (CSF) and participates in the blood-CSF barrier. It is essential for brain immunosurveillance and the clearance of toxics, and for brain development and activity. Indeed, the CP secretes a large variety of trophic factors in the CSF that impact the entire brain. These factors are mainly implicated in neurogenesis, but also in the maintenance of brain functions and the vasculature. In this mini-review, we provide an overview of the various trophic factors secreted by the CP in the CSF, and describe their roles in the developing, adult and diseased brain.
Similar content being viewed by others
References
Alshehri B, D’Souza D G, Lee J Y, Petratos S, Richardson S J (2015). The diversity of mechanisms influenced by transthyretin in neurobiology: development, disease and endocrine disruption. J Neuroendocrinol, 27(5): 303–323
Ashpole N M, Sanders J E, Hodges E L, Yan H, Sonntag WE (2015). Growth hormone, insulin-like growth factor-1 and the aging brain. Exp Gerontol, 68: 76–81
Aurbach E L, Inui E G, Turner C A, Hagenauer M H, Prater K E, Li J Z, Absher D, Shah N, Blandino P, Bunney W E, Myers R M, Barchas J D, Schatzberg A F, Watson S J, Akil H (2015). Fibroblast growth factor 9 is a novel modulator of negative affect. Proc Natl Acad Sci USA, 112(38): 11953–11958
Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, Amit I, Schwartz M (2014). Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science, 346(6205): 89–93
Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N (2003). Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci, 24(3): 623–631
Binder D K, Scharfman H E (2004). Brain-derived neurotrophic factor. Growth Factors, 22(3): 123–131
Brinker T, Stopa E, Morrison J, Klinge P (2014). A new look at cerebrospinal fluid circulation. Fluids Barriers CNS, 11 (1): 10
Budni J, Bellettini-Santos T, Mina F, Garcez M L, Zugno A I (2015). The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis, 6(5): 331–341
Carro E, Trejo J L, Spuch C, Bohl D, Heard J M, Torres-Aleman I (2006). Blockade of the insulin-like growth factor I receptor in the choroid plexus originates Alzheimer’s-like neuropathology in rodents: new cues into the human disease? Neurobiol Aging, 27 (11): 1618–1631
Chen C P C, Chen R L, Preston J E (2012). The influence of ageing in the cerebrospinal fluid concentrations of proteins that are derived from the choroid plexus, brain, and plasma. Exp Gerontol, 47(4): 323–328
Cheng Y, Black I B, Di Cicco-Bloom E (2002). Hippocampal granule neuron production and population size are regulated by levels of bFGF. Eur J Neurosci, 15(1): 3–12
Chodobski A, Szmydynger-Chodobska J (2001). Choroid plexus: target for polypeptides and site of their synthesis. Microsc Res Tech, 52(1): 65–82
Craig C G, Tropepe V, Morshead C M, Reynolds B A, Weiss S, van der Kooy D (1996). In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci, 16(8): 2649–2658
Damkier H H, Brown P D, Praetorius J (2013). Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev, 93(4): 1847–1892
Das K P, Chao S L, White L D, Haines W T, Harry G J, Tilson H A, Barone S (2001). Differential patterns of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 mRNA and protein levels in developing regions of rat brain. Neuroscience, 103 (3): 739–761
Delgado A C, Ferrón S R, Vicente D, Porlan E, Perez-Villalba A, Trujillo C M, D’Ocón P, Fariñas I (2014). Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron, 83(3): 572–585
Doetsch F, Petreanu L, Caille I, Garcia-Verdugo J M, Alvarez-Buylla A (2002). EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron, 36(6): 1021–1034
Dziegielewska K M, Ek J, Habgood M D, Saunders N R (2001). Development of the choroid plexus. Microsc Res Tech, 52(1): 5–20
Emerich D F, Skinner S J M, Borlongan C V, Vasconcellos A V, Thanos C G (2005). The choroid plexus in the rise, fall and repair of the brain. BioEssays, 27(3): 262–274
Emerich D F, Vasconcellos AV, Elliott R B, Skinner S J, Borlongan C V (2004). The choroid plexus: function, pathology and therapeutic potential of its transplantation. Expert Opin Biol Ther, 4(8): 1191–1201
Engelhardt B, Wolburg-Buchholz K, Wolburg H (2001). Involvement of the choroid plexus in central nervous system inflammation. Microsc Res Tech, 52(1): 112–129
Falcão A M, Marques F, Novais A, Sousa N, Palha J A, Sousa J C (2012). The path from the choroid plexus to the subventricular zone: go with the flow! Front Cell Neurosci, 6: 34
Falk S, Wurdak H, Ittner L M, Ille F, Sumara G, Schmid M T, Draganova K, Lang K S, Paratore C, Leveen P, Suter U, Karlßson S, Born W, Ricci R, Götz M, Sommer L (2008). Brain area-specific effect of TGF-ß signaling on Wnt-dependent neural stem cell expansion. Cell Stem Cell, 2(5): 472–483
Forlenza O V, Diniz B S, Teixeira A L, Radanovic M, Talib L L, Rocha N P, Gattaz W F (2015). Lower cerebrospinal fluid concentration of brain-derived neurotrophic factor predicts progression from mild cognitive impairment to Alzheimer’s disease. Neuromolecular Med, 17(3): 326–332
Gao L, Zhou S, Cai H, Gong Z, Zang D (2014). VEGF levels in CSF and serum in mild ALS patients. J Neurol Sci, 346(1-2): 216–220
Gato A, Alonso MI, Martín C, Carnicero E, Moro J A, De la Mano A, Fernández J M, Lamus F, Desmond M E. Embryonic cerebrospinal fluid in brain development: neural progenitor control. Croat Med J, 55(4): 299–305
Gong Z, Gao L, Guo J, Lu Y, Zang D (2015). bFGF in the CSF and serum of sALS patients. Acta Neurol Scand, 132(3): 171–178
González-Marrero I, Giménez-Llort L, Johanson C E, Carmona-Calero EM, Castañeyra-Ruiz L, Brito-Armas JM, Castañeyra-Perdomo A, Castro-Fuentes R (2015). Choroid plexus dysfunction impairs betaamyloid clearance in a triple transgenic mouse model of Alzheimer’s disease. Front Cell Neurosci, 9: 17
Greenwood S, Swetloff A, Wade A M, Terasaki T, Ferretti P (2008). Fgf2 is expressed in human and murine embryonic choroid plexus and affects choroid plexus epithelial cell behaviour. Cerebrospinal Fluid Res, 5 (1): 20
Hébert J M, Mishina Y, McConnell S K (2002). BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron, 35(6): 1029–1041
Huang S L, Shi W, Jiao Q, He X J (2011). Change of neural stem cells in the choroid plexuses of developing rat. Int J Neurosci, 121(6): 310–315
Huang X, Ketova T, Fleming J T, Wang H, Dey S K, Litingtung Y, Chiang C (2009). Sonic hedgehog signaling regulates a novel epithelial progenitor domain of the hindbrain choroid plexus. Development, 136(15): 2535–2543
Iliff J J, Wang M, Liao Y, Plogg B A, Peng W, Gundersen G A, Benveniste H, Vates G E, Deane R, Goldman S A, Nagelhus E A, Nedergaard M (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid ß. Sci Transl Med, 4 (147): 147ra111
Itokazu Y, Kitada M, Dezawa M, Mizoguchi A, Matsumoto N, Shimizu A, Ide C (2006). Choroid plexus ependymal cells host neural progenitor cells in the rat. Glia, 53(1): 32–42
Jackson E L, Garcia-Verdugo J M, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A (2006). PDGFR a- positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron, 51(2): 187–199
Jin K, Sun Y, Xie L, Batteur S, Mao X O, Smelick C, Logvinova A, Greenberg D A (2003). Neurogenesis and aging: FGF-2 and HBEGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell, 2(3): 175–183
Jin K, Zhu Y, Sun Y, Mao X O, Xie L, Greenberg D A (2002). Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA, 99(18): 11946–11950
Johanson C, McMillan P, Tavares R, Spangenberger A, Duncan J, Silverberg G, Stopa E (2004). Homeostatic capabilities of the choroid plexus epithelium in Alzheimer’s disease. Cerebrospinal Fluid Res, 1 (1): 3
Johanson C, Stopa E, Baird A, Sharma H (2011a). Traumatic brain injury and recovery mechanisms: peptide modulation of periventricular neurogenic regions by the choroid plexus-CSF nexus. J Neural Transm (Vienna), 118(1): 115–133
Johanson C, Stopa E, McMillan P, Roth D, Funk J, Krinke G (2011b). The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol Pathol, 39(1): 186–212
Johansson P A (2014). The choroid plexuses and their impact on developmental neurogenesis. Front Neurosci, 8: 340
Krizhanovsky V, Ben-Arie N (2006). A novel role for the choroid plexus in BMP-mediated inhibition of differentiation of cerebellar neural progenitors. Mech Dev, 123(1): 67–75
Krzyzanowska A, Carro E (2012). Pathological alteration in the choroid plexus of Alzheimer’s disease: implication for new therapy approaches. Front Pharmacol, 3: 75
Kunis G, Baruch K, Rosenzweig N, Kertser A, Miller O, Berkutzki T, Schwartz M (2013). IFN-g-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain, 136 (Pt 11): 3427–3440
Lehtinen MK, Zappaterra MW, Chen X, Yang Y J, Hill A D, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, D’Ercole A J, Wong E T, La Mantia A S, Walsh C A (2011). The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron, 69(5): 893–905
Li Y, Chen J, Chopp M (2002). Cell proliferation and differentiation from ependymal, subependymal and choroid plexus cells in response to stroke in rats. J Neurol Sci, 193(2): 137–146
Licht T, Eavri R, Goshen I, Shlomai Y, Mizrahi A, Keshet E (2010). VEGF is required for dendritogenesis of newly born olfactory bulb interneurons. Development, 137(2): 261–271
Liddelow S A (2015). Development of the choroid plexus and blood- CSF barrier. Front Neurosci, 9: 32
Lun M P, Monuki E S, Lehtinen M K (2015). Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci, 16(8): 445–457
Mackenzie F, Ruhrberg C (2012). Diverse roles for VEGF-A in the nervous system. Development, 139(8): 1371–1380
Maharaj A S R, Walshe T E, Saint-Geniez M, Venkatesha S, Maldonado A E, Himes N C, Matharu K S, Karumanchi S A, D’Amore P A (2008). VEGF and TGF-ß are required for the maintenance of the choroid plexus and ependyma. J Exp Med, 205 (2): 491–501
Maisonpierre P C, Belluscio L, Friedman B, Alderson R F, Wiegand S J, Furth ME, Lindsay RM, Yancopoulos G D (1990). NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron, 5(4): 501–509
Marques F, Sousa J C, Coppola G, Gao F, Puga R, Brentani H, Geschwind D H, Sousa N, Correia-Neves M, Palha J A (2011). Transcriptome signature of the adult mouse choroid plexus. Fluids Barriers CNS, 8 (1): 10
Mashayekhi F, Azari M, Moghadam L M, Yazdankhah M, Naji M, Salehi Z (2009). Changes in cerebrospinal fluid nerve growth factor levels during chick embryonic development. J Clin Neurosci, 16 (10): 1334–1337
Mashayekhi F, Sadeghi M, Rajaei F (2011). Induction of perlecan expression and neural cell proliferation by FGF-2 in the developing cerebral cortex: an in vivo study. J Mol Neurosci, 45(2): 87–93
McCoy MK, Tansey MG (2008). TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation, 5 (1): 45
Meeker R B, Williams K, Killebrew D A, Hudson L C (2012). Cell trafficking through the choroid plexus. Cell Adhes Migr, 6(5): 390–396
Mesquita S D, Ferreira A C, Gao F, Coppola G, Geschwind D H, Sousa J C, Correia-Neves M, Sousa N, Palha J A, Marques F (2015). The choroid plexus transcriptome reveals changes in type I and II interferon responses in a mouse model of Alzheimer’s disease. Brain Behav Immun, 49: 280–292
Miyan J A, Nabiyouni M, Zendah M(2003). Development of the brain: a vital role for cerebrospinal fluid. Can J Physiol Pharmacol, 81(4): 317–328
Moore L, Bain J M, Loh J M, Levison S W (2014). PDGF-responsive progenitors persist in the subventricular zone across the lifespan. ASN Neuro, 6(2): 65–81
Nielsen C M, Dymecki S M (2010). Sonic hedgehog is required for vascular outgrowth in the hindbrain choroid plexus. Dev Biol, 340 (2): 430–437
Nilsson C, Hultberg B M, Gammeltoft S (1996). Autocrine role of insulin-like growth factor II secretion by the rat choroid plexus. Eur J Neurosci, 8(3): 629–635
Pencea V, Bingaman K D, Freedman L J, Luskin M B (2001). Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol, 172(1): 1–16
Pillai A, Kale A, Joshi S, Naphade N, Raju M S V K, Nasrallah H, Mahadik S P (2010). Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol, 13(4): 535–539
Prasongchean W, Vernay B, Asgarian Z, Jannatul N, Ferretti P (2015). The neural milieu of the developing choroid plexus: neural stem cells, neurons and innervation. Front Neurosci, 9: 103
Preston J E (2001). Ageing choroid plexus-cerebrospinal fluid system. Microsc Res Tech, 52(1): 31–37
Rabie M A, Mohsen M, Ibrahim M, El-Sawy Mahmoud R (2014). Serum level of brain derived neurotrophic factor (BDNF) among patients with bipolar disorder. J Affect Disord, 162: 67–72
Redzic Z B, Preston J E, Duncan J A, Chodobski A, Szmydynger- Chodobska J (2005). “The Choroid Plexus-Cerebrospinal Fluid System: From Development to Aging, ” in Current Topics in Developmental Biology, ed. Gerald P. Schatten (Academic Press), 1–52. Available at: http://www.sciencedirect.com/science/article/pii/ S0070215305710012 [Accessed November 15, 2013].
Redzic Z B, Segal M B (2004). The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv Drug Deliv Rev, 56(12): 1695–1716
Ruiz de Almodovar C, Coulon C, Salin P A, Knevels E, Chounlamountri N, Poesen K, Hermans K, Lambrechts D, van Geyte K, Dhondt J, Dresselaers T, Renaud J, Aragones J, Zacchigna S, Geudens I, Gall D, Stroobants S, Mutin M, Dassonville K, Storkebaum E, Jordan B F, Eriksson U, Moons L, D’Hooge R, Haigh J J, Belin M F, Schiffmann S, van Hecke P, Gallez B, Vinckier S, Chédotal A, Honnorat J, Thomasset N, Carmeliet P, Meissirel C (2010). Matrix-binding vascular endothelial growth factor (VEGF) isoforms guide granule cell migration in the cerebellum via VEGF receptor Flk1. J Neurosci, 30(45): 15052–15066
Salehi Z, Mashayekhi F, Naji M, Pandamooz S (2009). Insulin-like growth factor-1 and insulin-like growth factor binding proteins in cerebrospinal fluid during the development of mouse embryos. J Clin Neurosci, 16(7): 950–953
Sathyanesan M, Girgenti M J, Banasr M, Stone K, Bruce C, Guilchicek E, Wilczak-Havill K, Nairn A, Williams K, Sass S, Duman J G, Newton S S (2012). A molecular characterization of the choroid plexus and stress-induced gene regulation. Transl Psychiatry, 2 (7): e139
Saunders N R, Daneman R, Dziegielewska K M, Liddelow S A (2013). Transporters of the blood-brain and blood-CSF interfaces in development and in the adult. Mol Aspects Med, 34(2-3): 742–752
Schänzer A, Wachs F P, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate K H, Kuhn H G (2004). Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol, 14(3): 237–248
Scola G, Andreazza A C (2015). The role of neurotrophins in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry, 56: 122–128
Segklia A, Seuntjens E, Elkouris M, Tsalavos S, Stappers E, Mitsiadis T A, Huylebroeck D, Remboutsika E, Graf D (2012). Bmp7 regulates the survival, proliferation, and neurogenic properties of neural progenitor cells during corticogenesis in the mouse. PLoS ONE, 7 (3): e34088
Serot J M, Béné M C, Foliguet B, Faure G C (1997). Altered choroid plexus basement membrane and epithelium in late-onset Alzheimer’s disease: an ultrastructural study. Ann N Y Acad Sci, 826(1 Cerebrovascul): 507–509
Serot J M, Foliguet B, Béné M C, Faure G C (2001). Choroid plexus and ageing in rats: a morphometric and ultrastructural study. Eur J Neurosci, 14(5): 794–798
Spatazza J, Lee H H C, Di Nardo A A, Tibaldi L, Joliot A, Hensch T K, Prochiantz A (2013). Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Reports, 3(6): 1815–1823
Spector R, Johanson C E (2010). Vectorial ligand transport through mammalian choroid plexus. Pharm Res, 27(10): 2054–2062
Spector R, Keep R F, Robert Snodgrass S, Smith Q R, Johanson C E (2015a). A balanced view of choroid plexus structure and function: Focus on adult humans. Exp Neurol, 267: 78–86
Spector R, Robert Snodgrass S, Johanson C E (2015b). A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp Neurol, 273: 57–68
Stolp H B, Molnár Z (2015). Neurogenic niches in the brain: help and hindrance of the barrier systems. Front Neurosci, 9: 20
Storkebaum E, Carmeliet P (2004). VEGF: a critical player in neurodegeneration. J Clin Invest, 113(1): 14–18
Strazielle N, Mutin M, Ghersi-Egea J F (2005). Les plexus choroïdes: une interface dynamique entre sang et liquide cephalo-rachidien. Morphologie, 89(285): 90–101
Strelau J, Sullivan A, Böttner M, Lingor P, Falkenstein E, Suter-Crazzolara C, Galter D, Jaszai J, Krieglstein K, Unsicker K (2000). Growth/differentiation factor-15/macrophage inhibitory cytokine-1 is a novel trophic factor for midbrain dopaminergic neurons in vivo. J Neurosci, 20(23): 8597–8603
Tochitani S, Kondo S (2013). Immunoreactivity for GABA, GAD65, GAD67 and Bestrophin-1 in the meninges and the choroid plexus: implications for non-neuronal sources for GABA in the developing mouse brain. PLoS ONE, 8 (2): e56901
Turner C A, Thompson R C, Bunney WE, Schatzberg A F, Barchas J D, Myers R M, Akil H, Watson S J (2014). Altered choroid plexus gene expression in major depressive disorder. Front Hum Neurosci, 8: 238
Vaccarino F M, Schwartz M L, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin J D, Wyland J J, Hung Y T (1999). Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci, 2(3): 246–253
Wagner J P, Black I B, Di Cicco-Bloom E (1999). Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci, 19(14): 6006–6016
Watanabe M, Kang Y J, Davies L M, Meghpara S, Lau K, Chung C Y, Kathiriya J, Hadjantonakis A K, Monuki E S (2012). BMP4 sufficiency to induce choroid plexus epithelial fate from embryonic stem cell-derived neuroepithelial progenitors. J Neurosci, 32(45): 15934–15945
Werner H, Le Roith D (2014). Insulin and insulin-like growth factor receptors in the brain: physiological and pathological aspects. Eur Neuropsychopharmacol, 24(12): 1947–1953
Xia Y X, Ikeda T, Xia X Y, Ikenoue T (2000). Differential neurotrophin levels in cerebrospinal fluid and their changes during development in newborn rat. Neurosci Lett, 280(3): 220–222
Zappaterra M W, Lehtinen M K (2012). The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cell Mol Life Sci, 69(17): 2863–2878
Ziegler A N, Levison SW, Wood T L (2015). Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat Rev Endocrinol, 11 (3): 161–170
Ziegler A N, Schneider J S, Qin M, Tyler WA, Pintar J E, Fraidenraich D, Wood T L, Levison S W (2012). IGF-II promotes stemness of neural restricted precursors. Stem Cells, 30(6): 1265–1276
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arnaud, K., Di Nardo, A.A. Choroid plexus trophic factors in the developing and adult brain. Front. Biol. 11, 214–221 (2016). https://doi.org/10.1007/s11515-016-1401-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-016-1401-7