Abstract
Background
Cannabis use disorder (CUD) is increasingly common and contributes to a range of health and social problems. Cannabidiol (CBD) is a non-intoxicating cannabinoid recognised for its anticonvulsant, anxiolytic and antipsychotic effects with no habit-forming qualities. Results from a Phase IIa randomised clinical trial suggest that treatment with CBD for four weeks reduced non-prescribed cannabis use in people with CUD. This study examines the efficacy, safety and quality of life of longer-term CBD treatment for patients with moderate-to-severe CUD.
Methods/Design
A phase III multi-site, randomised, double-blinded, placebo controlled parallel design of a 12-week course of CBD to placebo, with follow-up at 24 weeks after enrolment.
Two hundred and fifty adults with moderate-to-severe CUD (target 20% Aboriginal), with no significant medical, psychiatric or other substance use disorders from seven drug and alcohol clinics across NSW and VIC, Australia will be enrolled.
Participants will be administered a daily dose of either 4 mL (100 mg/mL) of CBD or a placebo dispensed every 3-weeks. All participants will receive four-sessions of Cognitive Behavioural Therapy (CBT) based counselling. Primary endpoints are self-reported cannabis use days and analysis of cannabis metabolites in urine. Secondary endpoints include severity of CUD, withdrawal severity, cravings, quantity of use, motivation to stop and abstinence, medication safety, quality of life, physical/mental health, cognitive functioning, and patient treatment satisfaction. Qualitative research interviews will be conducted with Aboriginal participants to explore their perspectives on treatment.
Discussion
Current psychosocial and behavioural treatments for CUD indicate that over 80% of patients relapse within 1–6 months of treatment. Pharmacological treatments are highly effective with other substance use disorders but there are no approved pharmacological treatments for CUD. CBD is a promising candidate for CUD treatment due to its potential efficacy for this indication and excellent safety profile. The anxiolytic, antipsychotic and neuroprotective effects of CBD may have added benefits by reducing many of the mental health and cognitive impairments reported in people with regular cannabis use.
Trial registration
Australian and New Zealand Clinical Trial Registry: ACTRN12623000526673 (Registered 19 May 2023).
Similar content being viewed by others
Introduction
Cannabis use disorder
Cannabis is the third most widely used drug in the world, after tobacco and alcohol, with an estimated 209 million persons, or 4.1% of the global adult population having used cannabis in the previous year (2020), Cannabis use has increased by 23 per cent between 2010 and 2020 [1]. Worryingly, between 9 and 22% of people who use cannabis will develop moderate or severe cannabis use disorder (CUD) [2] signifying ongoing cannabis use despite clinically significant impairment in health and social function [2]. The most recent global estimate suggests approximately 22.1 million persons met diagnostic criteria for CUD in 2016 (289.7 cases per 100,000 people) [3, 4].
CUD is associated with an increased risk of numerous psychosocial outcomes, including: (i) mental health problems (e.g., anxiety, depression, psychosis, suicide); (ii) physical illness (e.g. respiratory, cardiovascular disease, cancer); (iii) cognitive impairment (e.g., verbal learning, memory and attention); (iv) impaired brain development with prenatal or adolescent exposure; (v) social harms (e.g. crime, employment, parenting, financial impacts); and (vi) motor vehicle accidents [5].
Treatment for CUD
Existing treatments for CUD have modest outcomes. Reviews of psychosocial interventions (e.g. cognitive behavioural therapy (CBT), motivational enhancement therapy) [6] and acute withdrawal management [7] indicate that over 80% of patients relapse within 1–6 months of attempting treatment [8,9,10,11]. In substance use disorders other than CUD, treatment outcomes are generally optimised when combining medications with psychosocial interventions [12]. Despite examining a wide variety of medications, there are no registered pharmacotherapies for treating CUD [13,14,15].
There is increasing interest in the use of cannabinoid medications to treat CUD. Promising results have emerged in RCTs with delta-9-tetrahydrocannabinol (THC)-based medications (e.g., nabiximols [16], a 1:1 ratio of THC and CBD) and synthetic THC-based medications (e.g., dronabinol, nabilone) [16, 17]. However, many individuals may not be attracted to cannabinoid ‘agonist’ therapy with THC-based medications as they may have intoxicating, psychotogenic, anxiogenic and addictive properties. Thus, there is growing interest in the potential of non-intoxicating cannabinoids, such as CBD, in the treatment of CUD.
Cannabidiol and CUD
Cannabidiol (CBD) is one of the many cannabinoids found in the Cannabis sativa plant. It has diverse and multiple molecular targets [18] and anti-inflammatory, anxiolytic, anticonvulsant and antipsychotic properties [18,19,20,21,22,23,24]. Importantly in the context of CUD, CBD is a negative allosteric modulator of the activity of cannabinoid type 1 receptors within the central nervous system, restricting the ability of THC to bind to these receptors, thus reducing THC action [25]. CBD does not cause intoxication, dependence, or withdrawal on discontinuation, and does not result in positive results in urine or saliva tests used to detect cannabis use [26, 27]. Meta-analyses of clinical trials indicate CBD has a good safety profile [28], including in cannabis-using populations [29], and low oral bioavailability (approximately 6%) with a half-life of 18–32 h that permits once daily dosing [30].
CBD has shown promise in animal studies modelling addiction to a range of other substances, with reductions in self-administration of alcohol, opioids, cocaine, and methamphetamine [31]. Endocannabinoids are important regulators of the brain pathways that mediate neurodevelopment, drug-reward and addiction[32, 33]. In human studies examining other addictive drugs, CBD has been found to significantly reduce cue-induced craving and anxiety during abstinence from heroin [34] and cigarette use among tobacco smokers [35], but was not effective in reducing relapse or cravings in a placebo-controlled randomised trial for cocaine dependence [36].
There has been considerable scientific discussion in recent years about the promise of CBD as a treatment for CUD [37, 38], summarised in a recent review: “According to the previous evidence, it seems that CBD could play a crucial role in the management of CUD [38]”. Preclinical studies suggest that CBD administered to cannabis-dependent rodents reduces the severity of spontaneous withdrawal from THC [39,40,41]. Despite this, there has been little rigorous clinical research to date examining CBD as a treatment for CUD in humans. Early open-label case studies involving 10 participants with severe CUD indicate that CBD may ameliorate cannabis withdrawal severity and improve anxiety and sleep [39, 42, 43]. These studies used doses varying from 18 to 1200 mg daily but had methodological limitations that limit conclusions regarding efficacy.
A recent Phase 2a adaptive Bayesian RCT [44] demonstrated the promise of CBD for moderate-to-severe CUD and determined suitable doses for further investigations. 82 outpatients diagnosed with moderate-to-severe CUD were randomised to four-weeks of oral placebo (n = 23), 200 mg CBD (n = 12), 400 mg CBD (n = 24), or 800 mg CBD (n = 23), each receiving six sessions of motivational interviewing The 200 mg dose arm was eliminated as it was not efficacious following interim analyses. Both 400 mg and 800 mg groups were more efficacious than placebo in reducing cannabis use indicated by self-reported cannabis-free days and urinary carboxy-THC (THC-COOH), the inactive metabolite of THC excreted in urine. Doses were well tolerated with no serious adverse events. Reductions in cannabis use persisted 20-weeks after the four-week intervention in the 400 mg, but not the 800 mg group suggesting that further exploration of the 400 mg dose is warranted. However, the study was not powered to demonstrate efficacy, so larger RCTs are also necessary. The relatively high rates of relapse to heavy cannabis use at follow-up may be attributed to the brief treatment duration (4-weeks) examined in the phase 2a RCT. Indeed, in our previous 12-week RCT of nabiximols, we demonstrated that the full extent of reductions in cannabis use were not achieved until at least week 8 [16]. This suggests a prolonged duration, such as, 12-weeks of CBD and counselling may achieve better outcomes.
In their recent 12-week exploratory, observational, non-randomised, open-label study, Cleirec and colleagues [45] investigated the therapeutic potential of inhaled CBD amongst 20 patients, administered through an electronic vaping device, for the treatment of moderate-to-severe CUD. The average daily dose of inhaled CBD was 216 mg (equivalent to approximately 600–700 mg oral CBD). With a flexible dosing regimen and no formal counselling, the study demonstrated promising outcomes, including a notable 30% (n = 6) of participants achieving a 50% reduction in days of cannabis use, and 15% (n = 3) reporting complete abstinence by the end of the intervention. The absence of significant adverse events or the need for rescue medications further supports rigorous clinical trials examining the efficacy of CBD for CUD.
Fortin and colleagues [46] recently reported findings from an online anonymous survey of French residents who reported having used CBD within the past month. Respondents reported using CBD primarily to reduce their use of (illicit) cannabis. Of these, 59% (61/105) reported that their CBD use led to a large reduction in illegal cannabis consumption, 35% a moderate reduction, 6% no reduction, and 1% a moderate increase. While the study is limited by the self-report nature of the data and the inherent sampling biases of online surveys, the study provides a consumer perspective of the promise of CBD for treating CUD, complementing findings from the aforementioned preclinical and clinical studies.
In addition to CBD’s potential to facilitate a reduction in cannabis use, prolonged high-dose CBD usage in individuals with CUD may offer an additional advantage—potentially mitigating the adverse cognitive and mental health effects of long-term THC exposure [40, 41]. Animal studies indicate CBD reverses THC-induced memory deficits, conditioned place aversion and decreased social interaction [26]. In a human laboratory study, pre-treatment with CBD reduced acute THC-induced persecutory symptoms and hippocampal-dependent memory impairment [40]. In an open-label study, 10-weeks of daily oral CBD (200 mg) was associated with reduced cognitive deficits, psychotic-like and depressive symptoms and increased hippocampal volumes in chronic cannabis users (daily or near-daily use) (despite continued cannabis use), with the greatest benefits seen in those with cannabis dependence [47]. In the same trial, hippocampal and amygdala functional connectivity with other cortical regions (precentral and lingual gyrus, respectively), changed from pre-to-post intervention, with strong effect sizes (d > 1) [48]. However, in a Phase 2a RCT, CBD was not found to significantly impact cognition relative to placebo, except in the 800 mg group [49], although the study was underpowered. Nevertheless, the above studies suggest that the anxiolytic, antipsychotic and neuroprotective effects of CBD may improve the psychological, cognitive and brain health commonly related to long-term cannabis use [24]. This may be an added benefit of using high-dose CBD in people seeking treatment for CUD and identifies potential ‘secondary outcomes’ for future studies.
What are suitable primary endpoints for clinical trials of CUD treatment?
A challenge in embarking on clinical trials for substance use disorder is choosing a primary endpoint. Whilst historically abstinence (cessation of all use) has often been used in cannabis treatment research, there is increasing recognition that abstinence may not be the primary goal of treatment for patients who use cannabis. A recent consensus expert panel identified suitable outcomes when undertaking clinical trials for the treatment of CUD [50], recommending primary outcomes of (a) self-reported frequency of use, using the Time Line Follow Back (TLFB) method [51]; (b) biological assessment of cannabis use, with urinalysis of the metabolite THC-COOH or oral fluid detection of THC; and (c) severity of CUD, using a structured instrument measuring DSM-5 Criteria (e.g. Mini-International Neuropsychiatric Interview (MINI) [52]). In line with these recommendations, and consistent with the previous Phase 2a RCT, we propose to use two primary endpoints to measure illicit cannabis use: (1) self-reported ‘cannabis-free days’ and (2) urinary THC-COOH, across the 12-week treatment period of the study, alongside a range of secondary outcome measures.
Cannabis use in Indigenous Australian populations
Cannabis use is more prevalent among Aboriginal and Torres Strait Island people than non-Indigenous Australians. Data from the 2019 National Aboriginal and Torres Strait Islander Health Survey [53] indicated 24% (31% males, 18% females) of Indigenous people aged ≥ 15 years reported cannabis use in the past year, an increase from 19% in the corresponding 2012–2013 survey, and 30% higher than non-Indigenous Australians [53]. The elevated prevalence of illicit drug use among Indigenous Australians could be attributed to personal and familial factors, including intergenerational trauma from colonisation and experiences of racism. Societal-level influences such as persistent social and economic marginalisation contribute significantly to the increased likelihood of substance use amongst Indigenous Australians [1, 2].
Not only are prevalence rates of CUD higher, but the harms also resulting from cannabis use are greater in Indigenous Australian communities. Indigenous Australians are five times more likely to be hospitalised for CUD than non-Indigenous Australians [54] and six times more likely to seek treatment for cannabis use than non-Indigenous Australians when adjusted for age [2].
Yet despite the high prevalence of cannabis use and related harms in Indigenous Australians, to date, there have been no clinical treatment trials for CUD among Indigenous Australian populations. This study aims to ensure that a representative proportion of Indigenous Australian participants, with a target of 20% of the total sample, are recruited to the study. The target reflects the proportion of Indigenous Australian clients attending for cannabis treatment in participating study sites, with representation of Aboriginal researchers, health workers and consumers at all levels of the project governance (see Methods).
Summary
Cannabis Use Disorder poses significant health and social risks. Existing psychosocial treatments for CUD have modest effects, prompting the exploration of effective medications. CBD has emerged as a promising treatment for CUD, backed by preclinical evidence, and pilot data from a recent Phase 2a RCT. CBD offers additional advantages by potentially alleviating the mental health, and cognitive impairments associated with prolonged cannabis use.
Methods
Research hypothesis and study aims
The research hypothesis is that CBD, compared to placebo, will achieve statistically and clinically significant reductions in cannabis use, as measured by the number of self-reported cannabis-free days and urinary THC-COOH levels, among treatment-seeking patients with moderate-severe CUD.
The primary aim of the CBD-CUD study is to examine the efficacy of CBD, compared to placebo, in reducing cannabis use (as measured by self-report and quantitative measures of cannabis metabolites (THC-COOH) in urine drug screens) during treatment (Weeks 1–12) in participants seeking treatment for moderate-severe CUD, when used in combination with psychological interventions.
Secondary aims include examination of (i) safety, (ii) other cannabis related measures (e.g., cannabis withdrawal and cravings, cannabis-related problems); (iii) tobacco and other substance use; (iv) health and quality of life (QoL) measures, (v) patient experience measures; (vi) treatment retention rates; (vii) cognitive performance; (viii) post-treatment (Week 24) cannabis use, health outcomes and QoL measures.
Study design
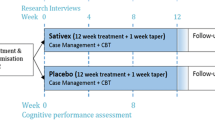
The study is a parallel group prospective double-blind Phase 3 randomised controlled trial comparing a 12-week treatment period of oral CBD (400 mg daily) (Experimental) to placebo (Control), with both groups receiving 4 sessions of manualised CBT-based counselling. Research interviews will be conducted at baseline (week 1), 3-weekly during the study intervention (weeks 4, 7, 10 and 13) and 12-weeks after the end of treatment (week 25) (Fig. 1). The study will use a modified intention-to-treat analysis. The expected number of participants is 250, of which we estimate approximately 20% (n = 50) will be of Indigenous background. The study will also include qualitative interviews with Indigenous Australian participants in both control and intervention groups (a total of n = 15–25 Indigenous Australian participants) to examine their experiences in the study.
Ethical statement
The study will be conducted in accordance with the National Statement on Ethical Conduct in Human Research (2007), the CPMP/ICH Note for Guidance on Good Clinical Practice and consistent with the principles that have their origin in the Declaration of Helsinki. The study was approved by the Sydney Local Health District Human Research Ethics Committee (no 2022/ETH02467) and the Aboriginal Health and Medical Research Council’s Human Research Ethics Committee (no 2110/23). The project has an Aboriginal Reference Group that oversees all aspects of the study, including data collection and analysis as they relate to Indigenous Australians. The study has been registered on the Australian and New Zealand Clinical Trial Registry (ACTRN12623000526673).
Setting and study sites
The multicenter trial will be coordinated from the Specialty of Addiction Medicine, Faculty of Medicine and Health, University of Sydney (study sponsor). Treatment will be provided at seven specialist addiction outpatient treatment centers: four in Sydney, one in Newcastle and two in Melbourne, Australia.
Participants and recruitment
Eligibility criteria
The target study population is treatment-seeking adults with moderate to severe CUD under conditions of informed consent. Eligibility will be assessed by an Addiction Medicine or Psychiatry credentialed Study Medical Officer (SMO).
Inclusion criteria:
-
1.
Aged 18 to 65 years.
-
2.
Meeting DSM-5 criteria for moderate or severe CUD (≥ 4/11 criteria) [2], with recent frequent cannabis use (≥ 4 days per week in the preceding 4 weeks).
-
3.
Willing and able to provide informed consent to study procedures.
-
4.
Proficient in English at a conversational level sufficient to participate in a counselling intervention.
Exclusion criteria aim to exclude individuals with conditions that jeopardise safety or confound data interpretation:
-
1
Prescribed medicinal cannabis products (e.g., CBD, THC) for any indication in the previous 4 weeks.
-
2
Another active (past year) moderate-severe substance use disorder other than tobacco; determined on clinical assessment using DSM-5 criteria.
-
3
Active or severe medical (e.g., pain, epilepsy, cardiovascular disease) or psychiatric (e.g., psychosis, severe affective disorder) conditions based on clinical assessment.
-
4
Moderate to severe hepatic disease (transaminase elevations > 3 times, bilirubin > 2 times upper normal limits at screening).
-
5
Pregnant or lactating women (based on urine β-hCG at screening).
-
6
Hypersensitivity to CBD or any excipients of Investigational Product.
-
7
Using medications with known drug-drug hepatic CYP-450 interactions with CBD: 3A4, (e.g., carbamazepine, fluvoxamine, methadone), 2C19 (e.g., rifampin); CYP2B6 (e.g., bupropion), CYP2C9 (e.g., warfarin).
-
8
Not available during treatment or follow-up (e.g., travel, impending residential detoxification or residential rehabilitation admission, impending imprisonment).
-
9
Court-mandated treatment requiring abstinence from drugs.
-
10
Current active (counselling and/or medication-based) treatment for CUD.
-
11
Received an investigational medicinal product within the last 4 weeks (or 5 half-lives if using long-acting investigational drugs).
Participant numbers
Sample size calculations are based on the analysis of the primary outcome, that is, the difference between placebo and CBD groups in total number of Cannabis-free Days over the 12-week intervention period. Ferguson has suggested that the minimum effect size (Cohen’s d) for an effect of practical clinical significance is 0.4167 [55]. To achieve 90% power (two-tailed) and α = 0.05, a total of N = 250 (n = 125 per group) participants are needed to detect a between-group effect size of d = 0.41. Of the target 250 sample, it is estimated approximately 20% (n = 50) of the study sample (N = 250) will be Indigenous Australians.
Participants who discontinue study procedures after commencing study interventions (medication dispensed on Day 1) will not be replaced in the study but will be included in the modified intention-to-treat analyses. Participants enrolled and randomised, but do not commence any treatment (no medication dispensed or other clinical interventions) will not be included in the final analysis.
Recruitment, screening and assessment
Participants will be recruited from people seeking treatment at participating study sites, and/or people interested in the study in response to study advertisements at local health services, social media, and clinical trial recruitment platforms. On initial contact with the service, potential participants will be informed of the study and if interested, referred to a site coordinator to complete telephone screening. Following telephone screening, potentially eligible participants will be scheduled a face-to face assessment with a Study Medical Officer (SMO) to confirm eligibility. Potential participants will sign a medical screen consent form prior to the SMO completing a structured history, clinical examination, and any laboratory investigations with the participants. The SMO will also explain the study requirements to the potential participant and explain the study medication and any potential side effects. Eligible participants are scheduled an appointment (Week 1, Day 1) to attend for enrolment into the study. For those participants who are not eligible or choose not to participate in the study, alternative treatment options will be organised in collaboration with the patient, as clinically appropriate.
Informed consent, randomisation and blinding
Written informed consent is obtained on Week 1, Day 1 of the study prior to the commencement of all subsequent study procedures. Consent is obtained with the site coordinator independent of treating clinicians, to minimise ‘pressure’ to participate in the study.
The randomisation schedule has been developed by an independent statistician, with eligible participants randomised in a 1:1 ratio between groups using variable block randomisation to help maintain blinding, with subjects stratified by (a) site (to achieve approximately equal numbers of active and placebo at each site) and (b) Indigenous Australian status (to allow direct between-group statistical comparisons within Indigenous Australian participants).
Participants, clinicians, and researchers involved in service delivery, data collection and analysis will remain blinded to study conditions using matched placebos manufactured by the same manufacturer. CBD and placebo will be packaged in identically labelled containers with the participant’s ID number and site. Aside from site trial pharmacists (who have no direct contact with participants), all other members of the clinical or research teams will be blinded to group allocation.
Unblinding will occur after all data are collected, entered, cleaned and the trial database has been locked. In circumstances where allocation needs to be unblinded (e.g. severe adverse event), the principal investigator will authorise the local site investigator to break the blind (via the site trial pharmacist).
Interventions
Medications
The experimental condition will receive 12 weeks of CBD oral 400 mg daily, administered as 200 mg liquid administered twice a day (BD). The CBD used in the trial is a plant-extracted pharmaceutical product (registered in Australia as Epidyolex® for the treatment of paediatric epilepsy), and is an oral liquid (clear, colourless to yellow solution) containing 100 mg per ml, dispensed in 105 ml bottles. The placebo is identical in composition and appearance (with the exception of the CBD). Both CBD and placebo are manufactured and supplied by Jazz Pharmaceuticals.
The dose is selected based on the findings of the Phase IIa RCT [29] that identified a daily dose of 400 mg CBD being more efficacious than placebo at reducing cannabis use during 4-week treatment and follow up.
Nicotine dependent participants will be offered smoking cessation counselling during the trial, with prescriptions and supply of nicotine replacement therapy (NRT) either in the form of 16-h topical patches (7, 14 or 21 mg) and/or nicotine chewing gum or lozenges provided.
Counselling
All participants will receive four structured 40–50-min counselling sessions over the 12-week medication phase, based on cognitive behavioural therapy (CBT) and motivation enhancement for relapse prevention, consistent with identified ‘best practice’ for cannabis cessation interventions [56]. Available evidence suggests 4-sessions of CBT deliver comparable outcomes to 6 or more sessions for treating CUD [57]. Counselling will be delivered by psychologists experienced in CUD treatment and trained to deliver manualised counselling interventions. Study Counsellors will keep a log of attendance at counselling sessions.
Clinical reviews
Participants will have 3-weekly medical reviews with the SMO over the 12-week intervention (Weeks 1, 4, 7, 10 and Week 13). At each appointment, the SMO will review cannabis and other substance use since the last appointment, other health and social issues, and client goals, complete Concomitant Medications and Adverse Events assessments, collect UDS, and supply medications dispensed by the trial pharmacist.
Outcome measures
The primary outcomes are illicit cannabis use during the 12-week intervention period, operationalised using two endpoints:
-
1)
Cannabis-free Days over the 12-week intervention period, producing a continuous measure between days 1 and 85. Details regarding number of days of cannabis use will be collected at each research interview (baseline week 1, weeks 4, 7, 10, week 13 and 25) using the Time Line Follow Back (TLFB) approach, a reliable and validated measure of cannabis use, particularly when combined with biological assays (e.g. UDS) and confidentially reported to independent researchers74.
-
2)
Urinary quantitative analysis of THC-COOH (creatinine adjusted). Urine samples will be collected at weeks 1, 4, 7, 10, 13 and 25, and analysed using liquid chromatography-tandem mass spectrometry (LCMS). As THC-COOH can remain ‘positive’ using qualitative thresholds (e.g. 20 ng/ml in LCMS assays) for more than 30 days after abstinence in chronic heavy cannabis users [58], we will analyse quantitative levels of THC-COOH (creatinine adjusted) to detect differences in cannabis use between the two study groups, replicating the approach used in the pilot RCT [29] (see below).
Secondary outcomes (Table 1) include a range of measures that relate to cannabis use (including rates of abstinence or reduced frequency of cannabis use, cannabis withdrawal and cravings, cannabis related problems, severity of CUD), safety (adverse events), health outcomes (including mental health, physical health, QoL), consumer experience of the medication, cognitive performance, other substance use and post-treatment outcomes (12 weeks after the intervention). The relationship between experiences of racial discrimination (using the modified Everyday Discrimination Scale) and outcomes for Indigenous Australians will also be explored.
Research interviews
The schedule of trial procedures and assessments for participants, including the timing of research and clinical interventions is shown in Table 2. Participants are scheduled to have interviews with researchers at 3-weekly intervals during the 12-week intervention (Weeks 1, 4, 7, 10 and 13), and again 12 weeks after the intervention (Week 25). These interviews will be face-to-face with a researcher, although they can be undertaken by telehealth if required. All data collected at researcher interviews will be entered directly into an electronic database, REDCap, and kept confidential from treating clinicians. Participants will be reimbursed with shopping vouchers for time, inconvenience, and expenses of attending research interviews [59].
Qualitative interviews with indigenous participants
To gain insights into the experience of Indigenous Australian participants, semi-structured in-depth interviews will be conducted by Aboriginal researchers (part of the study team) at around week seven with Indigenous participants in both control and interventions groups until data saturation occurs—estimated at 15 to 25 participants. These interviews will examine topics such as (a) how participants perceived their cannabis use and identified their treatment goals, and how participants are supported by their family and community; (b) how participants engage with the study treatment procedures (medication and counselling) providing insights into future implementation. The interviews will take approximately 40 to 60 min and be digitally recorded and transcribed. A yarning methodology will be used for the data collection and analysis [60].
Data management and monitoring
Confidentiality of participant data will be secured by removing all identifiable data and replacing it with a unique identifier. The principal investigator and coordinating researcher will have access to key files that link the unique identifier to identifiable data if unblinding is necessary.
Trial data will be electronically entered and stored on REDCap on the research drive of the University of Sydney, with regular data back-up. After the trial, the data will be stored for a minimum of 15 years in a secured study-specific folder on the research drive of the University of Sydney, and access to de-identified data will be considered upon request by the principal investigator.
An independent Data Safety and Monitoring Committee (IDSMC), comprising of an addiction medicine specialist, a statistician and clinical pharmacologist will oversee the safety monitoring of the trial, involving ongoing reviews of any adverse events arising from the administration of CBD (unblinded data). The committee will also monitor aspects of study integrity and design should any protocol changes need to be made.
Data analysis
All data analysis will be performed using Bayesian models instead of frequentist. Bayesian methods can quantify evidence for both effects and the absence of effects, are less prone to non-convergence (due to regularisation), and sample from a joint posterior distribution, hence no family- or experiment-wise correction of regression coefficients for multiple comparisons is necessary [61]. We will use a modified intention-to-treat approach for data analysis, with group membership fixed as the medication type (placebo vs CBD) participants receive on their first study day. Missing data will be imputed via hierarchical multiple imputation [62].
Primary outcomes
We will model the effects of CBD on number of cannabis free days (out of 84 days) via single-level Gaussian regression with the outcome regressed on the main covariate experimental group (placebo vs CBD). Number of cannabis-free days in the 28 days prior to baseline will be included as a covariate to control for variation in participants’ prior frequency of use entering the study. Two treatment factors that could plausibly influence the primary outcomes and which vary across participants will also be included as covariates: number of counselling sessions attended during the study period (count variable range 0–4), and whether or not NRT was taken (binary variable measured at baseline: did not undertake NRT vs undertook NRT). If residuals are distributed normally, we will report the results from this analysis. If residuals are not distributed normally, we will treat cannabis-free days/84 as a bounded count instead of a numeric variable and use aggregated binomial regression with a logit link.
We will model urinary THC-COOH levels (a continuous outcome, in ng/MoL) via random-slopes mixed-effects models with the group, time (6-level categorical ordered predictor; Weeks 1 (baseline), 4, 7, 10, 13), the group × time interaction, number of counselling sessions attended, and whether or not NRT was undertaken as the fixed factors, and participant ID as the random factor.
Secondary outcomes
All repeated measures of secondary outcomes will be modelled using the same approach as for urinary THC-COOH. That is, random slopes mixed-effects models for repeated measures regressions with group, time, the group × time interaction, number of counselling sessions attended, and whether or not NRT was taken as the fixed factors. These models will all be based on the generalised linear model, with link functions differing depending on the form of the outcome, as follows:
-
(a)
Numeric (e.g., PROMIS-29 scores, marijuana craving questionnaire scores): Gaussian regression with identity link function
-
(b)
Ordinal (e.g., motivation to change cannabis use): ordinal logistic regression with logit link function
-
(c)
Bounded count (e.g., severity of CUD) or binary (e.g., participant rating of group allocation): binomial logistic regression with logit link function
-
(d)
Unbounded count (e.g., adverse event count): negative binomial regression with log link function
See Table 1 for the form of each outcome measure.
Several secondary outcomes are single observations per individual. Group, number of counselling sessions attended, and whether or not NRT was taken will be the sole predictors in these models. Total abstinence from cannabis during weeks 10–13 (non-abstinent vs abstinent) and 50% increase in cannabis-free days during weeks 10–13 relative to cannabis-free days prior to baseline (< 50% reduction vs ≥ 50% reduction) will be modelled with binary logistic regression with logit link function, relative risk of adverse events during the trial period with negative binomial regression with log link function, hazard of treatment dropout via discrete-time hazard model with complementary log–log link function.
Statistical methods for Indigenous Australian focussed outcomes
Indigenous Australian and non-Indigenous participants will be compared on baseline participant characteristics (e.g., age, gender, frequency of substance use, scores on quality-of-life scales) via simple regression: Gaussian for continuous measures, Logistic for binary and count variables, and multinomial logistic for multilevel categorical data. For the main study analyses, comparing the frequency of illicit cannabis use between placebo and CBD groups, all participants will be pooled and included in main analyses, irrespective of Indigenous status. However, an additional regression will be performed where Indigenous status and the interaction between Indigenous status and the study drug (Placebo vs CBD), along with the primary predictor study drug, will be included in the regression.
The study will stratify randomisation according to Indigenous status, to achieve an approximately equal number of Indigenous Australian participants on active and placebo conditions, thus requiring no additional statistical procedures beyond those outlined in the previous paragraph.
The effect of the experience of discrimination on outcomes related to cannabis use disorder will be estimated via regressing various outcomes related to cannabis use on scores on the modified Everyday Discrimination Scale (m-EDS). Two regressions will be performed for each outcome, with a different version of the m-EDS as the primary predictor in each: (i) a continuous version of the scale (i.e., total score) and (ii) a three-level categorical version of the scale (no vs low vs moderate-to-high). The outcomes that the m-EDS will be regressed on will be: (i) (baseline characteristics (e.g., years of regular cannabis use, scores on quality-of-life scales), (ii), treatment engagement (e.g., treatment retention, number of counselling sessions) and (iii) outcomes during the trial (e.g., frequency of cannabis use, health measures). As described above, the type of regression will depend on the type of outcome: Gaussian for continuous, logistic for binary or bounded count, ordinal logistic for ordered categorical, and negative binomial for unbounded count. The effect of m-EDS on treatment retention will be estimated via Kaplan Meier plots discrete-time hazard model with complementary log–log link function.
Qualitative data analysis
The qualitative data collected during week seven of treatment, will be analysed by the Aboriginal investigators and the Aboriginal reference group. The data will initially be deductively coded into a) cannabis use, treatment goals and family and community support and b) experience on the study. Data will then be coded in three stages 1) open coding, 2) axial coding and 3) focused/selective coding [63]. After coding has been completed, the data from the deductive code b) experience on the study will be separated into treatment and control groups. The Aboriginal reference group will then analyse and discuss the data. Any divergences in treatment experiences will be explored. The themes identified will be discussed with the Aboriginal reference group to ensure appropriate interpretation with an Aboriginal lens.
Study governance
The multisite study will occur across the two most populous states in Australia. It will be coordinated through several governance structures, including an overarching Steering Committee (senior research staff and Study Investigators), a Consumer Advisory Group and an Aboriginal Reference Group.
The Consumer Advisory Group includes a Consumer Researcher (member of project team) and between 8 and 12 people with lived experience of cannabis use and treatment, who advise the project team on the study procedures (including recruitment strategies, treatment, and data collection procedures) and assist in interpretation of findings, and dissemination activities with community groups (e.g., lay summaries of study findings).
The Aboriginal Reference Group includes Aboriginal Investigators, research staff, representatives of Aboriginal Health Workers at participating sites, and representatives of Aboriginal Alcohol and Other Drug (AOD) workers in services not participating in the study (to provide independent community perspectives). The Aboriginal Reference and Consumer Advisory Groups will be consulted to interpret and disseminate study findings.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
UNODC: World Drug Report. In.; 2022.
AIHW: Alcohol, tobacco & other drugs in Australia. In. Canberra: Commonwealth of Australia: Australian Institute of Health and Welfare; 2023.
Degenhardt L, Charlson F, Ferrari A, Santomauro D, Erskine H, Mantilla-Herrara A, Whiteford H, Leung J, Naghavi M, Griswold M, et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5(12):987–1012.
WHO. The health and social effects of nonmedical cannabis use. Geneva: World Health Organisation; 2016.
Teesson M, Slade T, Swift W, Mills K, Memedovic S, Mewton L, Grove R, Newton N, Hall W. Prevalence, correlates and comorbidity of DSM-IV Cannabis Use and Cannabis Use Disorders in Australia. Aust N Z J Psychiatry. 2012;46(12):1182–92.
Cooper K, Chatters R, Kaltenthaler E, Wong R. Psychological and psychosocial interventions for cannabis cessation in adults: a systematic review short report. Health Technol Assess. 2015;19(56):1–130.
Connor JP, Stjepanovic D, Budney AJ, Le Foll B, Hall WD. Clinical management of cannabis withdrawal. Addiction. 2022;117(7):2075–95.
Gates PJ, Sabioni P, Copeland J, Le Foll B, Gowing L. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;2016(5):CD005336.
Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, Rivas GR, Holland RM, Muhleisen P, Norberg MM, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiat. 2014;71(3):281–91.
Budney A, Radonovich K, Higgins S, Wong C. Adults seeking treatment for Marijuana dependence: a comparsion with cocaine-dependent treatment seekers. Exp Clin Psychopharmacol. 1998;6(4):419–26.
Copeland J, Swift W. Cannabis use disorder: epidemiology and management. Int Rev Psychiatry. 2009;21(2):96–103.
Danovitch I, Gorelick DA. State of the art treatments for cannabis dependence. Psychiatr Clin North Am. 2012;35(2):309–26.
Ray LA, Meredith LR, Kiluk BD, Walthers J, Carroll KM, Magill M. Combined pharmacotherapy and cognitive behavioral therapy for adults with alcohol or substance use disorders: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(6):e208279.
Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173–94.
Kondo KK, Morasco BJ, Nugent SM, Ayers CK, O’Neil ME, Freeman M, Kansagara D. Pharmacotherapy for the treatment of cannabis use disorder: a systematic review. Ann Intern Med. 2020;172(6):398–412.
Lintzeris N, Bhardwaj A, Mills L, Dunlop A, Copeland J, McGregor I, Bruno R, Gugusheff J, Phung N, Montebello M, et al. Nabiximols for the treatment of cannabis dependence: a randomized clinical trial. JAMA Intern Med. 2019;179(9):1242–53.
Allsop DJ, Lintzeris N, Copeland J, Dunlop A, McGregor IS. Cannabinoid replacement therapy (CRT): Nabiximols (Sativex) as a novel treatment for cannabis withdrawal. Clin Pharmacol Ther. 2015;97(6):571–4.
Publication of interim decisions proposing to amend, or not amend, the current Poisons Standard, September 2018 [https://www.tga.gov.au/resources/publication/scheduling-decisions-interim/publication-interim-decisions-amending-or-not-amending-current-poisons-standard-february-2019/11-nabiximols].
Arzimanoglou A, Brandl U, Cross JH, Gil-Nagel A, Lagae L, Landmark CJ, Specchio N, Nabbout R, Thiele EA, Gubbay O, et al. Epilepsy and cannabidiol: a guide to treatment. Epileptic Disord. 2020;22(1):1–14.
Bonaccorso S, Ricciardi A, Zangani C, Chiappini S, Schifano F. Cannabidiol (CBD) use in psychiatric disorders: A systematic review. Neurotoxicology. 2019;74:282–98.
McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, Taylor A, Wright S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225–31.
Millar SA, Stone NL, Bellman ZD, Yates AS, England TJ, O’Sullivan SE. A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol. 2019;85(9):1888–900.
Gulbransen G, Xu W, Arroll B. Cannabidiol prescription in clinical practice: an audit on the first 400 patients in New Zealand. BJGP Open. 2020;4(1):bjgpopen20X101010.
Osborne AL, Solowij N, Weston-Green K. A systematic review of the effect of cannabidiol on cognitive function: relevance to schizophrenia. Neurosci Biobehav Rev. 2017;72:310–24.
Elsaid S, Le Foll B. The complexity of pharmacology of cannabidiol (CBD) and its implications in the treatment of brain disorders. Neuropsychopharmacology. 2020;45(1):229–30.
McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Delta(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172(3):737–53.
Publication of interim decisions proposing to amend, or not amend, the current Poisons Standard, September 2018 [https://www.tga.gov.au/book-page/15-cannabidiol-and-tetrahydrocannabinols-thcs].
Taylor L, Crockett J, Tayo B, Checketts D, Sommerville K. Abrupt withdrawal of cannabidiol (CBD): a randomized trial. Epilepsy Behav. 2020;104(Pt A):106938.
Chesney E, Oliver D, Green A, Sovi S, Wilson J, Englund A, Freeman TP, McGuire P. Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology. 2020;45(11):1799–806.
Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84(11):2477–82.
Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99(12):8384–8.
Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77(2):299–318.
Epidyolex (Cannabidiol). In. Edited by Pharmaceuticals J. Kent, UK: Jazz Pharmaceuticals; 2024.
Chye Y, Christensen E, Solowij N, Yucel M. The endocannabinoid system and cannabidiol’s promise for the treatment of substance use disorder. Front Psychiatry. 2019;10:63.
Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, Oprescu AM, Salsitz E. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019;176(11):911–22.
Morgan CJ, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav. 2013;38(9):2433–6.
Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, Farrell M, Degenhardt L. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(12):995–1010.
Hindocha C, Freeman TP, Schafer G, Gardner C, Bloomfield MAP, Bramon E, Morgan CJA, Curran HV. Acute effects of cannabinoids on addiction endophenotypes are moderated by genes encoding the CB1 receptor and FAAH enzyme. Addict Biol. 2020;25(3):e12762.
Pokorski I, Clement N, Phung N, Weltman M, Fu S, Copeland J. Cannabidiol in the management of inpatient cannabis withdrawal: clinical case series. Future Neurol. 2017;12(3):133–40.
Broyd SJ, van Hell HH, Beale C, Yucel M, Solowij N. Acute and chronic effects of cannabinoids on human cognition-a systematic review. Biol Psychiatry. 2016;79(7):557–67.
Freeman AM, Petrilli K, Lees R, Hindocha C, Mokrysz C, Curran HV, Saunders R, Freeman TP. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neurosci Biobehav Rev. 2019;107:696–712.
Shannon S, Opila-Lehman J. Cannabidiol oil for decreasing addictive use of marijuana: a case report. Integr Med Clin J. 2015;14(6):31–5.
Crippa JA, Hallak JE, Machado-de-Sousa JP, Queiroz RH, Bergamaschi M, Chagas MH, Zuardi AW. Cannabidiol for the treatment of cannabis withdrawal syndrome: a case report. J Clin Pharm Ther. 2013;38(2):162–4.
Freeman TP, Hindocha C, Baio G, Shaban NDC, Thomas EM, Astbury D, Freeman AM, Lees R, Craft S, Morrison PD, et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry. 2020;7(10):865–74.
Cleirec G, Desmier E, Lacatus C, Lesgourgues S, Braun A, Peloso C, Obadia C. Efficiency of inhaled cannabidiol in cannabis use disorder: the pilot study cannavap. Front Psychiatry. 2022;13:899221.
Fortin D, Di Beo V, Massin S, Bisiou Y, Carrieri P, Barre T. A “Good” Smoke? The off-label use of cannabidiol to reduce cannabis use. Front Psychiatry. 2022;13:829944.
Solowij N, Broyd SJ, Beale C, Prick JA, Greenwood LM, van Hell H, Suo C, Galettis P, Pai N, Fu S, et al. Therapeutic effects of prolonged cannabidiol treatment on psychological symptoms and cognitive function in regular cannabis users: a pragmatic open-label clinical trial. Cannabis Cannabinoid Res. 2018;3(1):21–34.
Lorenzetti V, McTavish E, Broyd S, van Hell H, Ganella E, Kottaram AR, Beale C, Martin J, Galettis P, Solowij N et al. Daily cannabidiol administration for 10 weeks modulates hippocampal and amygdalar resting-state functional connectivity in cannabis users: a functional magnetic resonance imaging open-label clinical trial. Cannabis Cannabinoid Res. 2023.
Lees R, Hines LA, Hindocha C, Baio G, Shaban NDC, Stothart G, Mofeez A, Morgan CJA, Curran HV, Freeman TP. Effect of four-week cannabidiol treatment on cognitive function: secondary outcomes from a randomised clinical trial for the treatment of cannabis use disorder. Psychopharmacology. 2023;240(2):337–46.
Loflin MJE, Kiluk BD, Huestis MA, Aklin WM, Budney AJ, Carroll KM, D’Souza DC, Dworkin RH, Gray KM, Hasin DS, et al. The state of clinical outcome assessments for cannabis use disorder clinical trials: a review and research agenda. Drug Alcohol Depend. 2020;212:107993.
Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119(1–2):123–9.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33 quiz 34-57.
AIHW: Alcohol and other drug treatment services in Australia annual report - 2019. In. Canberra: Commonwealth of Australia: Australian Institue of Health and Welfare; 2020.
Whetton S, Trait R, J, Chrzanowska A, Donnelly N, McEntee A, Mukhtar A, Zahra E, Campbell G, Degenhardt L, Dey T et al. Quantifying the Social Costs of Cannabis Use to Australia in 2015/16. In. Perth, WA: National Drug Research Institute, Curtin University; 2020.
Ferguson CJ. An effect size primer: a guide for clinicians and researchers. Prof Psychol Res Pract. 2009;40(5):532–8.
Stone J, Marsh A, Dale A, Willis L, O’Toole S, Helfgott S, Bennetts A, Cleary L, Ditchburn S, Jacobson H, et al. Counselling Guidelines: Alcohol and other drug issues. Mental Health Commission: Perth; 2019.
Sabioni P, Le Foll B. Psychosocial and pharmacological interventions for the treatment of cannabis use disorder. Focus (Am Psychiatr Publ). 2019;17(2):163–8.
Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–94.
Fry CL, Ritter A, Baldwin S, Bowen KJ, Gardiner P, Holt T, Jenkinson R, Johnston J. Paying research participants: a study of current practices in Australia. J Med Ethics. 2005;31(9):542–7.
Bessarab D, Ng’Andu B. Yarning about yarning as a legitimate method in Indigenous research. Int J Crit Indig Stud. 2010;3(1):37–50.
Kruschke JK. Doing Bayesian Data Analysis: A tutorial with R, JAGS, and Stan. In: Inc E, editor. Front Matter. 2nd ed. Bloomington: Indiana University; 2015. p. 530–50.
van Buuren S: Flexible imputation of missing data. Stef Van Buuren. , 2 edn: Chapman & Hall Books; 2018.
Corbin J, Strauss A. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21.
Norberg MM, Mackenzie J, Copeland J. Quantifying cannabis use with the timeline followback approach: a psychometric evaluation. Drug Alcohol Depend. 2012;121(3):247–52.
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. In.; 2013.
Heishman S, J, Singleton E, G, Liguori A: Marijuana Craving Questionnaire: Development and initial validation of a self-report instrument. In., vol. 96. Addiction; 2001.
Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol. 2000;68(5):898–908.
Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: a test of the relapse prevention model. J Consult Clin Psychol. 1994;62(1):92–9.
Ryan A, Holmes J, Hunt V, Dunlop A, Mammen K, Holland R, Sutton Y, Sindhusake D, Rivas G, Lintzeris N. Validation and implementation of the Australian Treatment Outcomes Profile in specialist drug and alcohol settings. Drug Alcohol Rev. 2014;33(1):33–42.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86(9):1119–27.
Mason OJ, Morgan CJ, Stefanovic A, Curran HV. The psychotomimetic states inventory (PSI): measuring psychotic-type experiences from ketamine and cannabis. Schizophr Res. 2008;103(1–3):138–42.
The PTSD Checklist for _DSM-5_ (PCL-5). [www.ptsd.va.gov].
Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, Rowland CR. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health and Quality of Life Outcomes. 2004;2(12).
Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology. 2013;227(1):177–92.
Thurber KA, Colonna E, Jones R, Gee GC, Priest N, Cohen R, Williams DR, Thandrayen J, Calma T, Lovett R et al. Prevalence of everyday discrimination and relation with wellbeing among Aboriginal and Torres Strait Islander adults in Australia. Int J Environ Res Public Health. 2021;18(12).
van der Linde I, Horsman L, Bright P. The validity of abbreviated forms of the National Adult Reading Test and Spot-the-Word 2 for estimating full-scale IQ. Neuropsychol Rehabil. 2022;32(10):2534–43.
Rey A. L’examen psychologique dans les cas d’encephopathie traumatique. Archives de Psychologie. 1941;28:286–340.
Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception Psychophysics. 1974;16(1):143–9.
Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55(4):352–8.
D W: Wechsler Adult Intelligence Scale - Third Edition (WAIS-III), 3 edn. San Antonio: The Psychological Corporation; 1997.
Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19(5):393–4.
Funding
This study was funded by National Health and Medical Research Council (NHMRC), Australia, project grant ID #2014980. Study drug (cannabidiol and placebo) was provided free of charge by Jazz Pharmaceuticals, UK.
Author information
Authors and Affiliations
Contributions
The study design was conceived of and designed by NL, LM, MD, LM, MM, SA, PH, DL, PM, MH, AD and TF. AB contributed to revisions in the study design, overall coordination of study start-up with all stakeholders and sites, and drafting the manuscript, tables and figures. AS has contributed to database design and set-up and VL has contributed to revisions in the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval and consent to participate: Sydney Local Health District Human Research Ethics Committee, Ref: 2022/ETH02467 on 03 February 2023. The Aboriginal Health and Medical Research Council’s Human Research Ethics Committee, Ref: 2110/23 on 30 May 2023. The sites: South East Sydney Local Health District, Sydney Local Health District, Northern Sydney Local Health District, Western Sydney Local Health District, Hunter New England Local Health District and Eastern Health, Turning Point will receive site-specific ethics approval.
All participants will sign a Participant Information Consent Form (PICF) before being enrolled in the study. Informed consent involves a 3-step process. (1) Verbal information about the trial is provided to the participant either face-to-face or over a telephone during the initial screen. (2) Participants eligible for a medical screen will be asked to sign a ‘medical screening consent form’ before seeing the study medical officer. (3) If the participant is deemed eligible by the study medical officer and the medical officer/ site coordinator has verbally discussed the trial to the participant and any queries or concerns addressed, the participant will sign the PICF on day 1 of commencing the trial.
Consent for publication
The participant information consent form includes a section relating to consent for de-identified data to be published. This is however not applicable for this paper.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bhardwaj, A.K., Mills, L., Doyle, M. et al. A phase III multisite randomised controlled trial to compare the efficacy of cannabidiol to placebo in the treatment of cannabis use disorder: the CBD-CUD study protocol. BMC Psychiatry 24, 175 (2024). https://doi.org/10.1186/s12888-024-05616-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-024-05616-3