Abstract
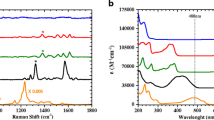
Sunitinib malate (SM) is a chemical compound belonging to the class of kinase inhibitors and is mainly used in cancer treatment. However, despite its effectiveness, SM can cause multiple side effects, including fatigue, nausea, vomiting, and diarrhea, which can affect the patient’s quality of life. Therefore, rapid and accurate diagnosis of SM is very important in many fields, especially for human health. Surface-enhanced Raman spectroscopy (SERS), an improved method for identifying biological and chemical substances with very low concentrations, is fast, reliable, and accurate. In order to identify the SM drug, filter paper substrates covered with silver nanoparticles (AgNPs) were used as SERS biosensors. First, AgNPs were prepared using a chemical reduction method. Then, the characteristics of the produced AgNPs were examined using FE-SEM, TEM, XRD, AFM, and UV–Vis analyses. The AgNPs were then coated on the substrates to create plasmonic active sites for identifying molecular vibrations of the SM drug. By coating the SM drug onto these substrates, the AgNPs reacted with the SM drug, successfully identifying trace amounts of the drug. The detection limit of the SERS plasmonic substrates for identifying the SM drug was 10−10 M. The average RSD for six repeated measurements was calculated at 5.39%. The enhancement factor for identifying molecular vibrations of the SM drug was experimentally calculated at 2.741 × 105 and numerically as 2.504 × 105. Therefore, the results indicate that these substrates are suitable for identifying low concentrations of this drug and can be used for drug monitoring purposes.






Similar content being viewed by others
Data Availability
The data related to the analyses are available from the corresponding author on reasonable request.
References
Vo-Dinh T (1998) Surface-enhanced Raman spectroscopy using metallic nanostructures. TrAC, Trends Anal Chem 17(8–9):557–582
Fan M, Andrade GF, Brolo AG (2020) A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal Chim Acta 1097:1–29
Schlücker S (2014) Surface-Enhanced raman spectroscopy: concepts and chemical applications. Angew Chem Int Ed 53(19):4756–4795
Wallace GQ, Masson J-F (2020) From single cells to complex tissues in applications of surface-enhanced Raman scattering. Analyst 145(22):7162–7185
Eskandari V, Hadi A, Sahbafar H (2022) Coating gold nanoparticles to a glass substrate by spin-coat method as a surface-enhanced raman spectroscopy (SERS) plasmonic sensor to detect molecular vibrations of bisphenol-a (BPA). ADVANCES IN NANO RESEARCH 13(5):417–426
Eskandari V et al (2022) Detection of molecular vibrations of atrazine by accumulation of silver nanoparticles on flexible glass fiber as a surface-enhanced Raman plasmonic nanosensor. Opt Mater 128:112310
Eskandari V et al (2023) Surface-enhanced Raman scattering (SERS) filter paper substrates decorated with silver nanoparticles for the detection of molecular vibrations of acyclovir drug. Spectrochim Acta Part A Mol Biomol Spectrosc 298:122762
Eskandari V et al (2022) Coating of silver nanoparticles (AgNPs) on glass fibers by a chemical method as plasmonic surface-enhanced Raman spectroscopy (SERS) sensors to detect molecular vibrations of Doxorubicin (DOX) drug in blood plasma. Arab J Chem 15(8):104005
Eskandari V et al (2023) Liposomes/nanoliposomes and surfaced-enhanced raman scattering (SERS): a review. Vibrat Spectroscop p. 103536
Eskandari V, Sahbafar H, Hadi A (2022) A review of surface-enhanced raman biosensors for studying different biological analytes and chemicals. Journal of Lasers in Medicine 18(4):57–57
Eskandari V et al (2022) A review of applications of surface-enhanced raman spectroscopy laser for detection of biomaterials and a quick glance into its advances for COVID-19 investigations. ISSS Journal of Micro and Smart Systems 11(2):363–382
Rezapour-Nasrabad R (2021) Feasibility of providing Namaste managed care to the elderly with Alzheimer’s disease. Archivos Venezolanos de Farmacologia y Terapéutica 40(4):455–463
Yang B et al (2019) Recent development of SERS technology: semiconductor-based study. ACS Omega 4(23):20101–20108
Xu F et al (2017) Prepare poly-dopamine coated graphene@ silver nanohybrid for improved surface enhanced Raman scattering detection of dyes. Sens Actuators, B Chem 243:609–616
Lai H et al (2019) Uniform arrangement of gold nanoparticles on magnetic core particles with a metal-organic framework shell as a substrate for sensitive and reproducible SERS based assays: application to the quantitation of Malachite Green and thiram. Microchim Acta 186:1–9
Han XX et al (2017) Semiconductor-enhanced Raman scattering: active nanomaterials and applications. Nanoscale 9(15):4847–4861
Lai H et al (2018) Recent progress on graphene-based substrates for surface-enhanced Raman scattering applications. Journal of Materials Chemistry B 6(24):4008–4028
Lai H et al (2020) Metal–organic frameworks: opportunities and challenges for surface-enhanced Raman scattering–a review. Journal of Materials Chemistry C 8(9):2952–2963
Xu K et al (2019) Toward flexible surface-enhanced Raman scattering (SERS) sensors for point-of-care diagnostics. Advanced Science 6(16):1900925
Restaino SM, White IM (2019) A critical review of flexible and porous SERS sensors for analytical chemistry at the point-of-sample. Anal Chim Acta 1060:17–29
Tay LL et al (2021) Paper-based surface-enhanced Raman spectroscopy sensors for field applications. J Raman Spectrosc 52(2):563–572
Eskandari V et al (2022) A review of paper-based substrates as surface-enhanced raman spectroscopy (SERS) biosensors and microfluidic paper-based SERS platforms. J Comput Appl Mech 53(1):142–156
Ngo YH et al (2012) Gold nanoparticle–paper as a three-dimensional surface enhanced Raman scattering substrate. Langmuir 28(23):8782–8790
Zhao B et al (2016) Silver dendrites decorated filter membrane as highly sensitive and reproducible three dimensional surface enhanced Raman scattering substrates. Appl Surf Sci 387:431–436
Kong L et al (2020) Highly enhanced Raman scattering with good reproducibility observed on a flexible PI nanofabric substrate decorated by silver nanoparticles with controlled size. Appl Surf Sci 511:145443
Dong J et al (2020) Flexible and transparent Au nanoparticle/graphene/Au nanoparticle ‘sandwich’substrate for surface-enhanced Raman scattering. Materials Today Nano 9:100067
Ogundare SA, van Zyl WE (2019) A review of cellulose-based substrates for SERS: fundamentals, design principles, applications. Cellulose 26:6489–6528
Huang C-C, Cheng C-Y, Lai Y-S (2020) based flexible surface enhanced Raman scattering platforms and their applications to food safety. Trends Food Sci Technol 100:349–358
Wei WY, White IM (2012) A simple filter-based approach to surface enhanced Raman spectroscopy for trace chemical detection. Analyst 137(5):1168–1173
Gotink KJ, Verheul HM (2010) Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis 13:1–14
Aparicio-Gallego G et al (2011) New insights into molecular mechanisms of sunitinib-associated side effects. Mol Cancer Ther 10(12):2215–2223
Motzer RJ et al (2006) Sunitinib in patients with metastatic renal cell carcinoma. JAMA 295(21):2516–2524
Adams VR, Leggas M (2007) Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors. Clin Ther 29(7):1338–1353
Raymond E et al (2011) Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364(6):501–513
Santoni M et al (2015) Treatment-related fatigue with sorafenib, sunitinib and pazopanib in patients with advanced solid tumors: an up-to-date review and meta-analysis of clinical trials. Int J Cancer 136(1):1–10
Jin J et al (2023) Sunitinib resistance in renal cell carcinoma: from molecular mechanisms to predictive biomarkers. Drug Resist Updat p. 100929
Morais C (2014) Sunitinib resistance in renal cell carcinoma. Journal of Kidney Cancer and VHL 1(1):1
Schwandt A et al (2009) Management of side effects associated with sunitinib therapy for patients with renal cell carcinoma. OncoTarg Therap p. 51–61
Kashani HM, Madrakian T, Afkhami A (2017) Highly fluorescent nitrogen-doped graphene quantum dots as a green, economical and facile sensor for the determination of sunitinib in real samples. New J Chem 41(14):6875–6882
Zhu X et al (2021) Efficacy and safety of individualized schedule of sunitinib by drug monitoring in patients with metastatic renal cell carcinoma. Cancer Manag Res p. 6833–6845
Baranowska I, Magiera S, Baranowski J (2013) Clinical applications of fast liquid chromatography: a review on the analysis of cardiovascular drugs and their metabolites. J Chromatogr B 927:54–79
Ranasinghe JC, Wang Z, Huang S (2022) Raman spectroscopy on brain disorders: transition from fundamental research to clinical applications. Biosensors 13(1):27
Zhou J, Zhong L (2022) Applications of liquid chromatography-mass spectrometry based metabolomics in predictive and personalized medicine. Front Mol Biosci 9:1049016
Štulı́k K, Pacáková V, Tichá M (2003) Some potentialities and drawbacks of contemporary size-exclusion chromatography. J Biochem Biophy Meth 56(1–3):1–13
Pinkston JD (2005) Advantages and drawbacks of popular supercritical fluid chromatography/mass spectrometry interfacing approaches—a user’s perspective. Eur J Mass Spectrom 11(2):189–197
Herrero P et al (2014) Comparison of triple quadrupole mass spectrometry and Orbitrap high-resolution mass spectrometry in ultrahigh performance liquid chromatography for the determination of veterinary drugs in sewage: benefits and drawbacks. J Mass Spectrom 49(7):585–596
Eskandari V et al (2023) A surface-enhanced Raman scattering (SERS) biosensor fabricated using the electrodeposition method for ultrasensitive detection of amino acid histidine. J Mol Struct 1274:134497
Rahimi M et al (2022) Design and fabrication of a differential MOEMS accelerometer based on Fabry-Pérot micro-cavities. IEEE Sens J 22(15):14779–14785
Foroughimehr N et al (2023) The effects of mmW and THz radiation on dry eyes: a finite-difference time-domain (FDTD) computational simulation using XFdtd. Sensors 23(13):5853
Yang P, Mittet R (2023) Controlled-source electromagnetic modeling using a high-order finite-difference time-domain method on a nonuniform grid. Geophysics 88(2):E53–E67
Sun G et al (2022) The finite-difference time-domain (FDTD) guided preparation of Ag nanostructures on Ti substrate for sensitive SERS detection of small molecules. Spectrochim Acta Part A Mol Biomol Spectrosc 269:120743
Beyene D et al (2018) Characterization of cellulase-treated fibers and resulting cellulose nanocrystals generated through acid hydrolysis. Materials 11(8):1272
Singh H et al (2018) Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artificial cells, nanomedicine, and biotechnology 46(6):1163–1170
Karooby E, Granpayeh N (2019) Potential applications of nanoshell bow-tie antennas for biological imaging and hyperthermia therapy. Opt Eng 58(6):065102–065102
Sood Y et al (2010) Surface charge of different paper making raw materials and its influence on paper properties
Villa JE, Santos DPD, Poppi RJ (2016) Fabrication of gold nanoparticle-coated paper and its use as a sensitive substrate for quantitative SERS analysis. Microchimica Acta 183:2745–2752
Mıhçıokur Ö, Özpozan T (2017) Molecular structure, vibrational spectroscopic analysis (IR & Raman), HOMO-LUMO and NBO analysis of anti-cancer drug sunitinib using DFT method. J Mol Struct 1149:27–41
Chen H-Y et al (2015) Large-scale hot spot engineering for quantitative SERS at the single-molecule scale. J Am Chem Soc 137(42):13698–13705
Granger JH et al (2016) Prospects for point-of-care pathogen diagnostics using surface-enhanced Raman scattering (SERS). Chem Soc Rev 45(14):3865–3882
Mandrile L et al (2019) Nondestructive Raman spectroscopy as a tool for early detection and discrimination of the infection of tomato plants by two economically important viruses. Anal Chem 91(14):9025–9031
Noroozi R et al (2023) 3D and 4D Bioprinting technologies: a game changer for the biomedical sector? Ann Biomed Eng 51(8):1683–1712
Yue S, Fang J, Xu Z (2022) Advances in droplet microfluidics for SERS and Raman analysis. Biosens Bioelectron 198:113822
Lussier F et al (2020) Deep learning and artificial intelligence methods for Raman and surface-enhanced Raman scattering. TrAC, Trends Anal Chem 124:115796
Acknowledgements
We would like to sincerely thank Mr. Hossein Sahbafar from the University of Tehran, Iran, for his invaluable assistance in performing the FDTD simulation presented in the “Enhancement Factor of SERS Substrates for the Detection of SM” section of the present study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Maryam Esmati. The first draft of the manuscript was written by Nima Hajari. Moreover, the whole investigation was supervised by Vahid Eskandari. Finally, all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Declaration of Generative AI and AI-assisted Technologies in the Writing Process
During the preparation of this work, the authors used ChatGPT, Sage, Bai Chat, Quillbot, and Grammarly in order to rephrase and edit the text grammatically and in terms of typo. After using this tools/services, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Conflict of Interest
The authors declare that there is no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Esmati, M., Hajari, N. & Eskandari, V. Flexible Plasmonic Paper Substrates as Surface-Enhanced Raman Scattering (SERS) Biosensors Enable Sensitive Detection of Sunitinib Malate Drug. Plasmonics 19, 21–31 (2024). https://doi.org/10.1007/s11468-023-01975-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-023-01975-x