Abstract
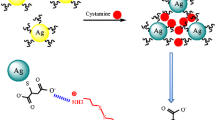
In the present study, a simple, facile, and highly selective colorimetric method is reported for cimetidine assay based on antiaggregation of in situ formed gold nanoparticles (AuNPs). The partially aggregated AuNPs were produced using ascorbic acid and chitosan in acidic media. The presence of cimetidine during formation of AuNPs led to the production of more dispersed nanoparticles resulting in a color change from blue to red. Consequently, monitoring of surface plasmon resonance absorbance of in situ formed AuNPs presents a reliable colorimetric probe for the determination of cimetidine. This plasmonic nanoprobe exhibits highly sensitive detection of cimetidine at concentrations as low as 2.7 ng mL−1 and is capable of quantitative assay of cimetidine over a range of 3.3–65.6 ng mL−1. The relative standard deviation corresponding to eight replicate determinations of 49.2 ng mL−1 of cimetidine was 4.1%. The method was highly selective toward many cations, anions, and sulfur-containing compounds. This sensing nanoprobe was effectively used for the rapid determination of cimetidine in tablet formulations and human serum samples.









Similar content being viewed by others
References
Winship DH (1978) Cimetidine in the treatment of duodenal ulcer: review. Gastroenterology 74:402–406
Lee S, Jung D, Kho Y, Ji K, Kim P, Ahn B, Choi K (2015) Ecotoxicological assessment of cimetidine and determination of its potential for endocrine disruption using three test organisms: Daphnia magna, Moina macrocopa, and Danio rerio. Chemosphere 135:208–216. https://doi.org/10.1016/j.chemosphere.2015.04.033
Deveney CW, Stein S, Way LW (1983) Cimetidine in the treatment of Zollinger-Ellison syndrome. Am J Surg 140:116–123. https://doi.org/10.1016/0002-9610(83)90271-4
Florey K (1985) Analytical profiles of drug substances. J Pharm Sci 74:907–908
Parsons ME, Ganellin CR (2006) Histamine and its receptors. Br J Pharmacol 147(1):S127–S135. https://doi.org/10.1038/sj.bjp.0706440
Kumar KG, Jayashree R (1993) Determination of cimetidine in pure form and in dosage forms using N- bromosuccinimide. J Pharm Biomed Anal 11:165–167. https://doi.org/10.1016/0731-7085(93)80137-P
Kelani KM, Aziz AM, Hegazy MA, Abdel Fattah L (2002) Different spectrophotometric methods for the determination of cimetidine, ranitidine hydrochloride, and famotidine. Spectrosc Lett 35(4):543–563. https://doi.org/10.1081/SL-120013890
Garcia S, Albero I, Sánchez-Pedrenõ C, Abuherba MS (2003) Spectrophotometric determination of cimetidine in pharmaceuticals and urine using batch and flow-injection methods. J Pharm Biomed Anal 32(4–5):1003–1010. https://doi.org/10.1016/S0731-7085(03)00202-4
Darwish IA, Hussein SA, Mahmoud AM, Hassan AI (2008) A sensitive spectrophotometric method for the determination of H2-receptor antagonists by means of N-bromosuccinimide and p-aminophenol. Acta Pharma 58(1):87–97. https://doi.org/10.2478/v10007-007-0047-z
Darwish IA, Hussein SA, Mahmoud AM, Hassan AI (2007) Spectrophotometric determination of H2-receptor antagonists via their oxidation with cerium(IV). Spectrochim Acta Part A 69(1):33–40. https://doi.org/10.1016/j.saa.2007.03.005
Iqbal T, Karyekar Ch S, Kinjo M, Ngan GC, Dowling Th C (2004) Validation of a simplified method for determination of cimetidine in human plasma and urine by liquid chromatography with ultraviolet detection. J Chromatogr B 799(2):337–341. https://doi.org/10.1016/j.jchromb.2003.10.030
Ashiru DAI, Patel R, Basit AW (2007) Simple and universal HPLC-UV method to determine cimetidine, ranitidine, famotidine and nizatidine in urine: application to the analysis of ranitidine and its metabolites in human volunteers. J Chromatogr B 860(2):235–240. https://doi.org/10.1016/j.jchromb.2007.10.029
Shafi N, Siddiqui FA, Naseem H, Sher N, Zubair A, Hussain A, Sial AA, Tasawer Baig M (2013) An overview of analytical determination of diltiazem, cimetidine, ranitidine, and famotidine by UV spectrophotometry and HPLC technique. J Chem 2013:1:1–1:116. https://doi.org/10.1155/2013/184948
Zendelovska D, Stafilov T (2003) Development of an HPLC method for the determination of ranitidine and cimetidine in human plasma following SPE. J Pharm Biomed Anal 33(2):165–173. https://doi.org/10.1016/S0731-7085(03)00265-6
Jantratid E, Prakongpan S, Oley JPF, Dressman JB (2007) Convenient and rapid determination of cimetidine in human plasma using perchloric acid-mediated plasma protein precipitation and high-performance liquid chromatography. Biomed Chromatogr 21(9):949–957. https://doi.org/10.1002/bmc.838
Mihaly GW, Cockbain S, Jones DB, Hanson RG, Smallwood RA (1982) High-pressure liquid chromatographic determination of cimetidine in plasma and urine. J Pharm Sci 71(5):590–592. https://doi.org/10.1002/jps.2600710528
Sousa CEMD, Tabosa MAM, Lima END, Filho JHD, Bedor DCG, Leal LB, Santana DPD (2014) Development of a simple and rapid method for the determination of cimetidine in human plasma by high performance liquid chromatography-mass spectrometry (HPLC-MS/MS): application to a bioequivalence study. Afr. J Pharm Pharmacol 8(46):1156–1163. https://doi.org/10.5897/AJPP2014.4155
Luo JW, Chen HW, He QH (2001) Determination of cimetidine in human plasma by use of coupled-flow injection, solid-phase extraction, and capillary zone electrophoresis. Chromatographia 53(5):295–300. https://doi.org/10.1007/BF02490427
Arrowood S, Hoyt AM (1991) Determination of cimetidine in pharmaceutical preparations by capillary zone electrophoresis. J Chromatogr 586(1):177–l80
Shamsipur M, Jalali F, Haghgoo S (2002) Preparation of a cimetidine ion-selective electrode and its application to pharmaceutical analysis. J Pharm Biomed Anal 27:867–872. https://doi.org/10.1016/0021-9673(91)80039-J
Huang X, El-Sayed MA (2010) Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res 1(1):13–28. https://doi.org/10.1016/j.jare.2010.02.002
Gupta RK, Ying G, Srinivasan MP, Lee PS (2012) Covalent assembly of gold nanoparticles: an application toward transistor memory. J Phys Chem B 116(32):9784–9790. https://doi.org/10.1021/jp3008283
RitheshRaj D, Prasanth S, Vineeshkumar TV, Sudarsanakumar C (2016) Surface plasmon resonance based fiber optic sensor for mercury detection using gold nanoparticles PVA hybrid. Opt Commun 367:102–107. https://doi.org/10.1016/j.optcom.2016.01.027
Deymehkar E, Taher MA, Karami C, Arman A (2018) Synthesis of SPR nanosensor using gold nanoparticles and its application to copper (II) determination. Silicon 10(4):1329–1336. https://doi.org/10.1007/s12633-017-9608-z
Salata OV (2004) Applications of nanoparticles in biology and medicine. J Nanobiotechnology 2(3):1–6. https://doi.org/10.1186/1477-3155-2-3
Fang W, Chen W, Yang J (2014) Colorimetric determination of DNA concentration and mismatches using hybridization-mediated growth of gold nanoparticle probes. Sens Actuator B 192:77–82. https://doi.org/10.1016/j.snb.2013.10.052
Rawat KA, Kailasa SK (2016) 4-Amino nicotinic acid mediated synthesis of gold nanoparticles for visual detection of arginine, histidine, methionine and tryptophan. Sens Actuator B 222:780–789. https://doi.org/10.1016/j.snb.2015.09.003
Attia AK, Badawy AM, Abd-Elhamid SG (2016) Determination of sparfloxacin and besifloxacin hydrochlorides using gold nanoparticles modified carbon paste electrode in micellar medium. RSC Adv 6:39605–39617. https://doi.org/10.1039/C6RA04851J
Zhang X, Zheng L, He Y (2016) Creatinine-modified gold nanoparticles for highly sensitive colorimetric sensing of nitroguanidine explosive. Plasmonics 11(6):1573–1578. https://doi.org/10.1007/s11468-016-0212-7
Wang C, Yu C (2013) Detection of chemical pollutants in water using gold nanoparticles as sensors: a review. Rev Anal Chem 32:1–14. https://doi.org/10.1515/revac-2012-0023
Jin Y, Li Z, Hu L, Shi X, Guan W, Du Y (2013) Synthesis of chitosan-stabilized gold nanoparticles by atmospheric plasma. Carbohydr Polym 91(1):152–156. https://doi.org/10.1016/j.carbpol.2012.08.018
Huang H, Yang X (2004) Synthesis of chitosan-stabilized gold nanoparticles in the absence/presence of tripolyphosphate. Biomacromolecules 5(6):2340–2346. https://doi.org/10.1021/bm0497116
Guan H, Yu J, Chi D (2013) Label-free colorimetric sensing of melamine based on chitosan- stabilized gold nanoparticles probes. Food Control 32:35–41. https://doi.org/10.1016/j.foodcont.2012.11.041
Chen Z, Wang Z, Chen X, Xu H, Liu J (2013) Chitosan-capped gold nanoparticles for selective and colorimetric sensing of heparin. J Nanopart Res 15:1930–1939. https://doi.org/10.1007/s11051-013-1930-9
Goia DV, Matijević E (1999) Tailoring the particle size of monodispersed colloidal gold. Colloid Surface A 146(1):139–152. https://doi.org/10.1016/S0927-7757(98)00790-0
Du CL, Yang MX, You YM, Chen T, Chen HY, Shen ZX (2009) Polymer-encapsulated silver nanoparticle monomer and dimer for surface-enhanced Raman scattering. Chem Phys Lett 473(4):317–320. https://doi.org/10.1016/j.cplett.2009.03.086
(2010) British Pharmacopoeia, vol 1. The Stationery Office Ltd., London, pp 501–502
Funding
The authors wish to thank Shahid Chamran University of Ahvaz for financial support of this project (Grant 1396).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rezaei, Z.B., Rastegarzadeh, S. & Kiasat, A. A Sensitive and Selective Colorimetric Probe for Cimetidine Determination Based on Surface Plasmon Resonance of Chitosan-Encapsulated Gold Nanoparticles. Plasmonics 14, 1169–1177 (2019). https://doi.org/10.1007/s11468-019-00905-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-019-00905-0