Abstract
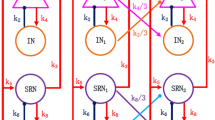
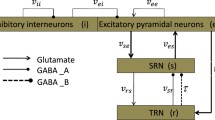
Based on a macroscopic mean-field model associating with the thalamus and cerebral cortex, we investigate how the newly proposed coordinated reset stimulation (CRS) strategy controls the absence seizures as some key parameters are changed. Different from the previous stimulation processes, CRS represents the intermittent pulse current administered to different structures including cortex, specific relay nuclei (SRN) and thalamus reticular nucleus (TRN) at different time by using three different micro-electrodes. In particular, we first simulate a typical absence epilepsy activity under the combined effect of the coupling strength between inhibitory interneurons (IIN)-excitatory pyramidal neurons (EPN) and EPN-TRN pathway. And then we explore the control mechanism of different parameters of 3:2 ON-OFF CRS on spike and slow-wave discharges (SWDs) region. Through analyzing the corresponding two-dimensional bifurcation diagrams, we find CRS is effective on controlling absence seizures in proper ranges of stimulation parameters. Especially, the combination of frequency and positive input duration can inhibit the pathological area more effectively. The obtained results might be helpful to study the pathophysiology mechanism of epilepsy, although the CRS’s feasibility still needs further exploration in clinical experiments.
Similar content being viewed by others
References
Fisher R S, Acevedo C, Arzimanoglou A, et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia, 2014, 55: 475–482
Buck D, Baker G A, Jacoby A, et al. Patients’ experiences of injury as a result of epilepsy. Epilepsia, 1997, 38: 439–444
Cockerell O C, Hart Y M, Sander J W A S, et al. Mortality from epilepsy: Results from a prospective population-based study. Lancet, 1994, 344: 918–921
Crunelli V, Leresche N. Childhood absence epilepsy: Genes, channels, neurons and networks. Nat Rev Neurosci, 2002, 3: 371–382
Timofeev I, Steriade M. Neocortical seizures: Initiation, development and cessation. Neuroscience, 2004, 123: 299–336
Breakspear M, Roberts J A, Terry J R, et al. A unifying explanation of primary generalized seizures through nonlinear brain modeling and bifurcation analysis. Cereb Cortex, 2005, 16: 1296–1313
Rodrigues S, Gonçalves J, Terry J R. Existence and stability of limit cycles in a macroscopic neuronal population model. Physica D, 2007, 233: 39–65
Fan D G, Wang Q Y. Synchronization and bursting transition of the coupled Hindmarsh-Rose systems with asymmetrical time-delays. Sci China Tech Sci, 2016, doi: 10.1007/s11431-016-0169-8
Marten F, Rodrigues S, Benjamin O, et al. Onset of polyspike complexes in a mean-field model of human electroencephalography and its application to absence epilepsy. Philos Trans R Soc A-Math Phys Eng Sci, 2009, 367: 1145–1161
Dehghani N, Peyrache A, Telenczuk B, et al. Dynamic balance of excitation and inhibition in human and monkey neocortex. Sci Rep, 2016, 6: 23176
van Vreeswijk C, Sompolinsky H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science, 1996, 274: 1724–1726
Shu Y, Hasenstaub A, McCormick D A. Turning on and off recurrent balanced cortical activity. Nature, 2003, 423: 288–293
Bonifazi P, Goldin M, Picardo M A, et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science, 2009, 326: 1419–1424
Dichter M A, Ayala G F. Cellular mechanisms of epilepsy: A status report. Science, 1987, 237: 157–164
Sillito A M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol, 1975, 250: 305–329
Florez C M, Lukankin V, Sugumar S, et al. Hypoglycemia-induced alterations in hippocampal intrinsic rhythms: Decreased inhibition, increased excitation, seizures and spreading depression. Neurobiol Dis, 2015, 82: 213–225
Puranam R S, He X P, Yao L, et al. Disruption of Fgf13 causes synaptic excitatory-inhibitory imbalance and genetic epilepsy and febrile seizures plus. J Neurosci, 2015, 35: 8866–8881
di Volo M, Burioni R, Casartelli M, et al. Neural networks with excitatory and inhibitory components: Direct and inverse problems by a mean-field approach. Phys Rev E, 2016, 93: 012305
Chen M, Guo D, Wang T, et al. Bidirectional control of absence seizures by the basal ganglia: A computational evidence. PLoS Comput Biol, 2014, 10: e1003495
Salam M T, Perez Velazquez J L, Genov R. Seizure suppression efficacy of closed-loop versus open-loop deep brain stimulation in a rodent model of epilepsy. IEEE Trans Neural Syst Rehabil Eng, 2016, 24: 710–719
Elliott R E, Morsi A, Tanweer O, et al. Efficacy of vagus nerve stimulation over time: Review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS >10 years. Epilepsy Behav, 2011, 20: 478–483
Morrell M. Brain stimulation for epilepsy: Can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol, 2006, 19: 164–168
Fan D, Wang Q. Improving desynchronization of parkinsonian neuronal network via triplet-structure coordinated reset stimulation. J Theor Biol, 2015, 370: 157–170
Tass P A, Qin L, Hauptmann C, et al. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann Neurol, 2012, 72: 816–820
Chabardès S, Kahane P, Minotti L, et al. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord, 2002, 4: 83–93
Morrell M J, Morrell M J. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology, 2011, 77: 1295–1304
Hu B, Wang Q Y. The conditions for onset of beta oscillations in an extended subthalamic nucleus-globus pallidus network. Sci China Tech Sci, 2014, 57: 2020–2027
Steyn-Ross M L, Steyn-Ross D A, Sleigh J W. Gap junctions modulate seizures in a mean-field model of general anesthesia for the cortex. Cogn Neurodyn, 2012, 6: 215–225
Jirsa V K, Haken H. Field theory of electromagnetic brain activity. Phys Rev Lett, 1996, 77: 960–963
Freyer F, Roberts J A, Becker R, et al. Biophysical mechanisms of multistability in resting-state cortical rhythms. J Neurosci, 2011, 31: 6353–6361
Chen M, Guo D, Li M, et al. Critical Roles of the Direct GABAergic pallido-cortical pathway in controlling absence seizures. PLoS Comput Biol, 2015, 11: e1004539
Wang H X, Wang Q Y, Zheng Y H. Bifurcation analysis for Hindmarsh-Rose neuronal model with time-delayed feedback control and application to chaos control. Sci China Tech Sci, 2014, 57: 872–878
Gu H G, Chen S G. Potassium-induced bifurcations and chaos of firing patterns observed from biological experiment on a neural pacemaker. Sci China Tech Sci, 2014, 57: 864–871
Mirski M A, Rossell L A, Terry J B, et al. Anticonvulsant effect of anterior thalamic high frequency electrical stimulation in the rat. Epilepsy Res, 1997, 28: 89–100
Velasco A L, Velasco F, Jiménez F, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox- Gastaut syndrome. Epilepsia, 2006, 47: 1203–1212
Usui N, Maesawa S, Kajita Y, et al. Suppression of secondary generalization of limbic seizures by stimulation of subthalamic nucleus in rats. J Neurosurg, 2005, 102: 1122–1129
Mina F, Benquet P, Pasnicu A, et al. Modulation of epileptic activity by deep brain stimulation: A model-based study of frequency-dependent effects. Front Comput Neurosci, 2013, 7: 94
Guo D, Wu S, Chen M, et al. Regulation of irregular neuronal firing by autaptic transmission. Sci Rep, 2016, 6: 26096
Guo D, Chen M, Perc M, et al. Firing regulation of fast-spiking interneurons by autaptic inhibition. Europhys Lett, 2016, 114: 30001
Roberts J A, Robinson P A. Modeling absence seizure dynamics: Implications for basic mechanisms and measurement of thalamocortical and corticothalamic latencies. J Theor Biol, 2008, 253: 189–201
Neiman A B, Yakusheva T A, Russell D F. Noise-induced transition to bursting in responses of paddlefish electroreceptor afferents. J Neurophysiol, 2007, 98: 2795–2806
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Z., Wang, Q. Effect of the coordinated reset stimulations on controlling absence seizure. Sci. China Technol. Sci. 60, 985–994 (2017). https://doi.org/10.1007/s11431-016-9043-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-016-9043-3