Abstract
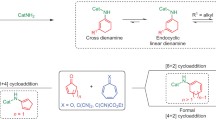
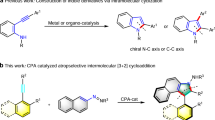
A chiral phosphoric acid catalyzed enantioselective [2 + 2] cycloaddition of alkynylindols or alkynylnaphthols with quinones is disclosed. A class of functionalized cyclobutenes with excellent yields, diastereo- and enantioselectivities were prepared under mild reaction conditions (70 examples, up to 99% yield, 99% ee, all > 50:1 dr). Mechanistic studies revealed that a dearomatization of indole or naphthol occurred to initiate the cycloaddition, followed by an intramolecular Michael addition with in situ generated allene-iminium or vinylidene-quinone methide intermediate. The competitive [2 + 3] cycloaddition was prevented in this catalytic system. An interesting central to axial chirality conversion via a rearrangement process was realized during transformation of the product.

Similar content being viewed by others
References
Carreira EM, Fessard TC. Chem Rev, 2014, 114: 8257–8322
Misale A, Niyomchon S, Maulide N. Acc Chem Res, 2016, 49: 2444–2458
Semmelhack MF, Tomoda S. J Am Chem Soc, 1981, 103: 2427–2428
Lee-Ruff E, Mladenova G. Chem Rev, 2003, 103: 1449–1484
Namyslo JC, Kaufmann DE. Chem Rev, 2003, 103: 1485–1538
Gauvry N, Lescop C, Huet F. Eur J Org Chem, 2006, 2006: 5207–5218
Xu Y, Conner ML, Brown MK. Angew Chem Int Ed, 2015, 54: 11918–11928
Frébault F, Luparia M, Oliveira M, Goddard R, Maulide N. Angew Chem Int Ed, 2010, 49: 5672–5676
Zhong C, Huang Y, Zhang H, Zhou Q, Liu Y, Lu P. Angew Chem Int Ed, 2020, 59: 2750–2754
Pirenne V, Traboulsi I, Rouvière L, Lusseau J, Massip S, Bassani DM, Robert F, Landais Y. Org Lett, 2020, 22: 575–579
Shibata T, Takami K, Kawachi A. Org Lett, 2006, 8: 1343–1345
Fan BM, Li XJ, Peng FZ, Zhang HB, Chan ASC, Shao ZH. Org Lett, 2010, 12: 304–306
Schotes C, Mezzetti A. Angew Chem Int Ed, 2011, 50: 3072–3074
Hu J, Yang Q, Yu L, Xu J, Liu S, Huang C, Wang L, Zhou Y, Fan B. Org Biomol Chem, 2013, 11: 2294–2301
Kossler D, Cramer N. Chem Sci, 2017, 8: 1862–1866
Jiao Z, Shi Q, Zhou JS. Angew Chem Int Ed, 2017, 56: 14567–14571
García-Morales C, Ranieri B, Escofet I, López-Suarez L, Obradors C, Konovalov AI, Echavarren AM. J Am Chem Soc, 2017, 139: 13628–13631
Parsutkar MM, Pagar VV, RajanBabu TV. J Am Chem Soc, 2019, 141: 15367–15377
Narasaka K, Hayashi Y, Shimadzu H, Niihata S. J Am Chem Soc, 1992, 114: 8869–8885
Takenaka Y, Ito H, Hasegawa M, Iguchi K. Tetrahedron, 2006, 62: 3380–3388
Ishihara K, Fushimi M. J Am Chem Soc, 2008, 130: 7532–7533
Enomoto K, Oyama H, Nakada M. Chem Eur J, 2015, 21: 2798–2802
Kang T, Ge S, Lin L, Lu Y, Liu X, Feng X. Angew Chem Int Ed, 2016, 55: 5541–5544
Zhang M, Wang XC. Angew Chem Int Ed, 2021, 60: 17185–17190
Maturi MM, Bach T. Angew Chem Int Ed, 2014, 53: 7661–7664
Golfmann M, Glagow L, Giakoumidakis A, Golz C, Walker JCL. Chem Eur J, 2023, 29: e202202373
Yang P, Jia Q, Song S, Huang X. Nat Prod Rep, 2023, 40: 1094–1129
Yu H, Dong S, Yao Q, Chen L, Zhang D, Liu X, Feng X. Chem Eur J, 2018, 24: 19361–19367
Zhong X, Tang Q, Zhou P, Zhong Z, Dong S, Liu X, Feng X. Chem Commun, 2018, 54: 10511–10514
Zhong X, Tan J, Qiao J, Zhou Y, Lv C, Su Z, Dong S, Feng X. Chem Sci, 2021, 12: 9991–9997
Xiao W, Ning L, Xin S, Dong S, Liu X, Feng X. Angew Chem Int Ed, 2022, 61: e202211596
Reetz M, List B, Jaroch S, Weinmann H. Organocatalysis. Heidelberg: Springer Science & Business, 2008
MacMillan DWC. Nature, 2008, 455: 304–308
Silvi M, Melchiorre P. Nature, 2018, 554: 41–49
Xiang SH, Tan B. Nat Commun, 2020, 11: 3786–3790
Moyano A, Rios R. Chem Rev, 2011, 111: 4703–4832
Pellissier H. Tetrahedron, 2012, 68: 2197–2232
Shi M, Wei Y, Zhao MX, Zhang J. Organocatalytic Cycloadditions for Synthesis of Carbo- and Heterocycles. Weinheim: Wiley-VCH, 2018
Engler TA, Letavic MA, Reddy JP. J Am Chem Soc, 1991, 113: 5068–5070
Brimble MA, Duncalf LJ, Reid DCW, Roberts TR. Tetrahedron, 1998, 54: 5363–5374
Engler TA, Letavic MA, Iyengar R, LaTessa KO, Reddy JP. J Org Chem, 1999, 64: 2391–2405
Zhou G, Corey EJ. J Am Chem Soc, 2005, 127: 11958–11959
Zheng H, Xu C, Wang Y, Kang T, Liu X, Lin L, Feng X. Chem Commun, 2017, 53: 6585–6588
Trofimov B, Sobenina LN, Stepanova Z, Ushakov IA, Sinegovskaya L, Vakul’skaya T, Mikhaleva AI. Synthesis, 2010, 2010(3): 470–476
Shoji T, Morita N, Maruyama M, Shimomura E, Maruyama A, Ito S, Yasunami M, Higashi J, Toyota K. Heterocycles, 2014, 88: 319–329
Swager T, Dengiz C. Synlett, 2017, 28: 1427–1431
Gou BB, Tang Y, Lin YH, Yu L, Jian QS, Sun HR, Chen J, Zhou L. Angew Chem Int Ed, 2022, 61
Yang H, Sun HR, He RQ, Yu L, Hu W, Chen J, Yang S, Zhang GG, Zhou L. Nat Commun, 2022, 13: 632–640
Wang LY, Yang L, Chen J, Zhou L. Synlett, 2023, 34: 1200–1214
Sweis RF, Schramm MP, Kozmin SA. J Am Chem Soc, 2004, 126: 7442–7443
Shen L, Zhao K, Doitomi K, Ganguly R, Li YX, Shen ZL, Hirao H, Loh TP. J Am Chem Soc, 2017, 139: 13570–13578
Bai YB, Luo Z, Wang Y, Gao JM, Zhang L. J Am Chem Soc, 2018, 140: 5860–5865
Zanini M, Cataffo A, Echavarren AM. Org Lett, 2021, 23: 8989–8993
Munakala A, Nallamilli T, Nanubolu JB, Chegondi R. Org Lett, 2022, 24: 892–896
Wang Y, Hu M, Ding L, Wang Y, Wang XN, Chang J. Org Lett, 2022, 24: 5056–5061
Sultan S, Bhat MS, Rizvi MA, Shah BA. J Org Chem, 2019, 84: 8948–8958
Álvarez-García J, Rubio-Pisabarro V, Silva-López C, Cid MM. Org Lett, 2020, 22: 4527–4531
Liao L, Shu C, Zhang M, Liao Y, Hu X, Zhang Y, Wu Z, Yuan W, Zhang X. Angew Chem Int Ed, 2014, 53: 10471–10475
Sun XX, Zhang HH, Li GH, Meng L, Shi F. Chem Commun, 2016, 52: 2968–2971
Gelis C, Bekkaye M, Lebée C, Blanchard F, Masson G. Org Lett, 2016, 18: 3422–3425
Liu QJ, Zhu J, Song XY, Wang L, Wang SR, Tang Y. Angew Chem Int Ed, 2018, 57: 3810–3814
Feng W, Yang H, Wang Z, Gou BB, Chen J, Zhou L. Org Lett, 2018, 20: 2929–2933
Akiyama T. Chem Rev, 2007, 107: 5744–5758
Terada M. Synthesis, 2010, 2010: 1929–1982
Parmar D, Sugiono E, Raja S, Rueping M. Chem Rev, 2014, 114: 9047–9153
Zhang X, Chen YH, Tan B. Tetrahedron Lett, 2018, 59: 473–486
See Supporting Information for details.
CCDC 2130886 (3d), 2175084 (3q), 2254781 (3as), 2174883 (6a′) and 2208602 (16b) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre
Xia ZL, Xu-Xu QF, Zheng C, You SL. Chem Soc Rev, 2020, 49: 286–300
Zi W, Zuo Z, Ma D. Acc Chem Res, 2015, 48: 702–711
Zheng C, You SL. Nat Prod Rep, 2019, 36: 1589–1605
Sheng FT, Wang JY, Tan W, Zhang YC, Shi F. Org Chem Front, 2020, 7: 3967–3998
Qin W, Liu Y, Yan H. Acc Chem Res, 2022, 55: 2780–2795
Liu H, Li K, Huang S, Yan H. Angew Chem Int Ed, 2022, 61: e202117063
Wang CS, Li TZ, Liu SJ, Zhang YC, Deng S, Jiao Y, Shi F. Chin J Chem, 2020, 38: 543–552
Kang Q, Zhao ZA, You SL. Org Lett, 2008, 10: 2031–2034
Gu Q, Rong ZQ, Zheng C, You SL. J Am Chem Soc, 2010, 132: 4056–4057
Liao S, Čorić I, Wang Q, List B. J Am Chem Soc, 2012, 134: 10765–10768
Feng J, Yan W, Wang D, Li P, Sun Q, Wang R. Chem Commun, 2012, 48: 8003–8005
Reid JP, Goodman JM. J Am Chem Soc, 2016, 138: 7910–7917
Melikian M, Gramüller J, Hioe J, Greindl J, Gschwind RM. Chem Sci, 2019, 10: 5226–5234
Santiago CB, Guo JY, Sigman MS. Chem Sci, 2018, 9: 2398–2412
Reid JP. Commun Chem, 2021, 4: 171–174
Li K, Huang S, Liu T, Jia S, Yan H. J Am Chem Soc, 2022, 144: 7374–7381
Helliwell M, Thomas EJ, Whitehouse DL. Synthesis, 2005, 2005: 3235–3238
George J, Sherburn MS. J Org Chem, 2019, 84: 14712–14723
Varlet T, Gelis C, Retailleau P, Bernadat G, Neuville L, Masson G. Angew Chem Int Ed, 2020, 59: 8491–8496
Nguyen TT. Org Biomol Chem, 2019, 17: 6952–6963
Yang H, Chen J, Zhou L. Chem Asian J, 2020, 15: 2939–2951
Min XL, Zhang XL, Shen R, Zhang Q, He Y. Org Chem Front, 2022, 9: 2280–2292
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC 21672170), the Natural Science Basic Research Plan in Shaanxi Province of China (2021JZ-40), and Shaanxi Fundamental Science Research Project for Chemistry & Biology (22JHQ007). The calculations were performed at Chemical HPC Center of NWU.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no conflict of interest.
Additional information
Supporting information
The supporting information is available online at chem.scichina.com and link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sun, HR., Yang, L., Li, Y. et al. Organocatalytic asymmetric [2 + 2] cycloaddition of alkynes with quinones. Sci. China Chem. 66, 2292–2299 (2023). https://doi.org/10.1007/s11426-023-1658-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-023-1658-9