Abstract
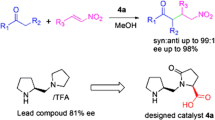
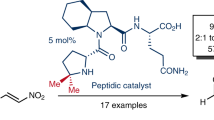
A new family of organocatalyst derived from proline has been developed and shown to be an efficient catalyst for asymmetric Michael addition of cyclohexanone to nitroolefins with high diastereo- and enanthio -selectivities. (syn: anti ratio up to 99:1, ee. up to 95%.). The result of computational studies at the B3LYP/6-31G* level indicate that both the hydrogen bonding and the stereo hindrance play the crucial role in the activation of the nitro alkene and help to discriminate between the two diastereofacial approaches.
Similar content being viewed by others
References
Dalko, P.I., Angew. Chem. Int. Ed. 2004, vol. 43, p. 5138. DOI: 10.1002/anie.200400650.
Perlmutter, A., Conjugative Additions in Organic Synthesis, Oxford Pergamon Press, 1992.
Ballini, R., Bosica, G., Fiorini, D., Palmieri, A., and Petrini, M., Chem. Rev. 2005, vol 105, p. 933. DOI: 10.1021/cr040602r.
Ono, N., The Nitro Group in Organic Synthesis New York: Wiley-VCH, 2001.
Berner, O.M., Tedeschi, L., and Enders, D., Eur. J. Org. Chem. 2002, p. 1877. DOI: 10.1002/1099-0690(200206)2002:12<1877::AIDEJOC1877> 3.0.CO;2-U.
Sakthive, K.I., Notz, W., Bui, T., and Barbas, C.F., J. Am. Chem.Soc. 2001, vol. 123, p. 5260. DOI: 10.1021/ja010037z.
List, B., Pojarliev, P., and Martin, H.J., Org. Lett. 2001, vol. 3, p. 2423. DOI: 10.1021/ol015799d.
Tsogoeva, S.B. and Wei, S. Chem. Commun. 2006, p. 1451. DOI: 10.1039/B517937H.
Huang, H. and Jacobsen, E.N., J. Am. Chem. Soc. 2006, vol. 128, p. 7170. DOI: 10.1021/ja0620890.
Xu, Y. and Cordova, A., Chem. Commun., 2006, p. 460. DOI: 10.1039/B514783M.
Luo, S., Mi, X., Zhang, L., Liu, S., Xu, H., and Cheng, J.-P., Angew. Chem., Int. Ed., 2006, vol. 45, p. 3093. DOI: 10.1002/anie.200600048.
Ishii, T., Fujioka, S., Sekiguchi, Y., and Kotsuki, H., J. Am. Chem. Soc., 2004, vol. 126, p. 9558. DOI: 10.1021/ja046871g.
Mase, N., Thayumanavan, R., Tanaka, F., and Barbas, C.F., Org. Lett., 2004, vol. 6, p. 2527. DOI: 10.1021/ol049196o.
Andrey, O., Alexakis, A., Tomassini, A., and Bernardinelli, G., Adv. Synth. Catal., 2004, vol. 346, p. 1147. DOI: 10.1002/adsc.200404037.
Cobb, A.J.A., Longbottom, D.A., Shaw, D.M., and Ley, S.V., Chem. Commun., 2004, p. 1808. DOI: 10.1039/B409646K.
Reyes, E., Vicario, J.L., Badia, D., and Carrillo, L., Org. Lett. 2006, vol. 8, p. 6135. DOI: 10.1021/ol062627d.
Cao, C.L., Ye, M.C., Sun, X.L., and Tang, Y., Org. Lett., 2006, vol. 8, p. 2901. DOI: 10.1021/ol060481c.
Wang, W., Wang, J., and Li, H., Angew. Chem. Int. Ed., 2005, vol. 44, p. 1369. DOI: 10.1002/anie.200461959.
Wang, J., Li, H., Lou, B., Zu, L., Guo, H., and Wang, W., Chem.-Eur. J. 2006, vol. 12, p. 4321. DOI: 10.1002/chem.200600115.
Pansare, S.V., and Pandya, K. J. Am. Chem. Soc., 2006, vol. 128, p. 9624. DOI: 10.1021/ja062701n.
Mase, N., Watanabe, K., Yoda, H., Takabe, K., Tanaka, F., and Barbas, C.F., J. Am. Chem. Soc., 2006, vol. 128, p. 4966. DOI: 10.1021/ja060338e.
Xu, D.-Q., Wang, L.-P., Luo, S.-P., Wang, Y.-F., Zhang, S., and Xu, Z.-Y., Eur. J. Org. Chem., 2008, p. 1049. DOI: 10.1002/ejoc.200700856.
Wang, B-G., Ma, B-C., Wang, Q., and Wang, W., Adv. Synth. Catal., 2010, vol. 352, p. 2923. DOI: 10.1002/adsc.201000508.
Almasi, D., Alonso, D.A., Bengoa, E.G., Nagel, Y., and Nàjera, C., Eur. J. Org. Chem., 2007, p. 2328. DOI: 10.1002/ejoc.200700031.
Zheng, Z., Perkins, B.L., Ni, Bukuo, J. Am. Chem. Soc., 2010, vol. 132, p. 50. DOI: 10.1021/ja9093583.
Albrecht, L., Jiang, H., and Jørgensen, K.A., Chem. Eur. J., 2014, vol. 20, p. 358. DOI: 10.1002/chem.201303982.
Zhang, R., Yin, G., Li, Y., Yan X., and Chen, L. RSC Adv., 2015, vol. 5, p. 3461. DOI: 10.1039/C4RA10684A.
Lu, D., Gong, Y., and Wang, W., Adv. Synth. Catal. 2010, vol. 352, p. 644. DOI: 10.1002/adsc.200900687.
Cao, X., Wang, G., Zhang, R., Wei, Y., Wang, W., Sun, H., and Chen, L., Org. Biomol. Chem. 2011, vol. 9, p. 6487. DOI: 10.1039/C1OB05679D.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Keith, T., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., and Fox, D.J., Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2013.
Becke, A.D., J. Chem. Phys. 1993, vol. 98, p. 1372. DOI: org/10.1063/1.464304.
Becke, A.D., J. Chem. Phys. 1993, vol. 98, p. 5648. DOI: org/10.1063/1.464913
Lee, C., Yang, W., and Parr, R.G., Phys. Rev. B 1988, vol. 37, p. 785. DOI: org/10.1103/PhysRevB.37.785.
Francisco, J.S. and Schlegel, H.B., J. Phys. Chem. 1988, vol. 88, p. 3736. DOI: org/10.1063/1.453873.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Yang, M., Zhang, Y.C., Zhao, J.Q. et al. The highly enantioselective bifunctional organocatalysts for the Michael addition of сyclohexanone to titroolefins. Russ J Gen Chem 86, 1381–1388 (2016). https://doi.org/10.1134/S1070363216060244
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363216060244