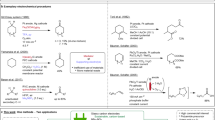
Abstract
A selective electrochemical oxidation was developed under mild condition. Various mono-carbonyl and multi-carbonyl compounds can be prepared from different aromatic hydrocarbons with moderate to excellent yield and selectivity by virtue of this electrochemical oxidation. The produced carbonyl compounds can be further transformed into α-ketoamides, homoallylic alcohols and oximes in a one-pot reaction. In particular, a series of α-ketoamides were prepared in a one-pot continuous electrolysis. Mechanistic studies showed that 2,2,2-trifluoroethan-1-ol (TFE) can interact with catalyst species and generate the corresponding hydrogen-bonding complex to enhance the electrochemical oxidation performance.

Similar content being viewed by others
References
Luque-Ortega JR, Reuther P, Rivas L, Dardonville C. J Med Chem, 2010, 53: 1788–1798
Nicolaou KC, Hale CRH, Nilewski C, Ioannidou HA. Chem Soc Rev, 2012, 41: 5185–5238
Hu QF, Zhou B, Huang JM, Gao XM, Shu LD, Yang GY, Che CT. J Nat Prod, 2013, 76: 292–296
Choudhary U, Mir AA, Emrick T. Macromolecules, 2017, 50: 3772–3778
Xie W, Wu Y, Zhang J, Mei Q, Zhang Y, Zhu N, Liu R, Zhang H. Eur J Med Chem, 2018, 145: 35–40
Ma S, Gu Z. Angew Chem Int Ed, 2005, 44: 7512–7517
Zhang Q, Yang F, Wu Y. Chem Commun, 2013, 49: 6837–6839
Ramgren SD, Garg NK. Org Lett, 2014, 16: 824–827
Tran PH, Hansen PE, Nguyen HT, Le TN. Tetrahedron Lett, 2015, 56: 612–618
Zhang C, Xu Z, Zhang L, Jiao N. Angew Chem Int Ed, 2011, 50: 11088–11092
Zhang C, Zhang L, Jiao N. Adv Synth Catal, 2012, 354: 1293–1300
Pieber B, Kappe CO. Green Chem, 2013, 15: 320–324
Li Q, Huang Y, Chen T, Zhou Y, Xu Q, Yin SF, Han LB. Org Lett, 2014, 16: 3672–3675
Huang Y, Chen T, Li Q, Zhou Y, Yin SF. Org Biomol Chem, 2015, 13: 7289–7293
Choudary BM, Prasad AD, Bhuma V, Swapna V. J Org Chem, 1992, 57: 5841–5844
Lounis Z, Riahi A, Djafri F, Muzart J. Appl Catal A-Gen, 2006, 309: 270–272
Verma S, Nasir Baig RB, Nadagouda MN, Varma RS. ACS Sustain Chem Eng, 2016, 4: 2333–2336
Rezaeifard A, Khoshyan A, Jafarpour M, Pourtahmasb M. RSC Adv, 2017, 7: 15754–15761
Urgoitia G, SanMartin R, Herrero MT, Domínguez E. Green Chem, 2011, 13: 2161–2166
Zhou Y, Long J, Li Y. Chin J Catal, 2016, 37: 955–962
Guo C, Zhang Y, Zhang Y, Wang J. Chem Commun, 2018, 54: 3701–3704
Karthikeyan P, Bhagat PR, Kumar SS. Chin Chem Lett, 2012, 23: 681–684
Ang WJ, Lam Y. Org Biomol Chem, 2015, 13: 1048–1052
Wu H, Song J, Xie C, Hu Y, Liu S, Han B. ACS Sustain Chem Eng, 2018, 6: 13670–13675
Napoly F, Kieffer R, Jean-Gérard L, Goux-Henry C, Draye M, Andrioletti B. Tetrahedron Lett, 2015, 56: 2517–2520
Miao C, Zhao H, Zhao Q, Xia C, Sun W. Catal Sci Technol, 2016, 6: 1378–1383
Li S, Zhu B, Lee R, Qiao B, Jiang Z. Org Chem Front, 2018, 5: 380–385
Han X, Zhou Z, Wan C, Xiao Y, Qin Z. Synthesis, 2013, 45: 615–620
Shaabani A, Keshipour S, Hamidzad M, Shaabani S. J Mol Catal A-Chem, 2014, 395: 494–499
Jafarpour M, Rezaeifard A, Yasinzadeh V, Kargar H. RSC Adv, 2015, 5: 38460–38469
Gaster E, Kozuch S, Pappo D. Angew Chem Int Ed, 2017, 56: 5912–5915
Araghi M, Bokaei F. Polyhedron, 2013, 53: 15–19
Shen D, Miao C, Wang S, Xia C, Sun W. Org Lett, 2014, 16: 1108–1111
Shaabani A, Hezarkhani Z, Badali E. RSC Adv, 2015, 5: 61759–61767
Rezaeifard A, Jafarpour M, Farrokhi A, Parvin S, Feizpour F. RSC Adv, 2016, 6: 64640–64650
Shaabani A, Borjian Boroujeni M, Laeini MS. Appl Organometal Chem, 2016, 30: 154–159
Ding ZD, Zhu W, Li T, Shen R, Li Y, Li Z, Ren X, Gu ZG. Dalton Trans, 2017, 46: 11372–11379
Wang W, Xu D, Sun Q, Sun W. Chem Asian J, 2018, 13: 2458–2464
Mečiarova M, Toma S, Heribanová A. Tetrahedron, 2000, 56: 8561–8566
Hashemi MM, Ghazanfari D, Karimi-Jaberi Z. Monatshefte fur Chem Chem Mon, 2004, 135: 185–188
Khan AT, Parvin T, Choudhury LH, Ghosh S. Tetrahedron Lett, 2007, 48: 2271–2274
Zhang J, Wang Z, Wang Y, Wan C, Zheng X, Wang Z. Green Chem, 2009, 11: 1973–1978
Kumar RA, Maheswari CU, Ghantasala S, Jyothi C, Reddy KR. Adv Synth Catal, 2011, 353: 401–410
Cui LQ, Liu K, Zhang C. Org Biomol Chem, 2011, 9: 2258–2265
Yin L, Wu J, Xiao J, Cao S. Tetrahedron Lett, 2012, 53: 4418–4421
Xu Y, Hu JT, Yan J. Chin Chem Lett, 2012, 23: 891–894
Badri R, Adlu M, Mohammadi MK. Arabian J Chem, 2015, 8: 62–65
Shaabani A, Laeini MS, Shaabani S, Seyyedhamzeh M. New J Chem, 2016, 40: 2079–2082
Ma Z, Zhang H, Yang Z, Ji G, Yu B, Liu X, Liu Z. Green Chem, 2016, 18: 1976–1982
Tan J, Zheng T, Yu Y, Xu K. RSC Adv, 2017, 7: 15176–15180
Hu Y, Zhou L, Lu W. Synthesis, 2017, 49: 4007–4016
Liu J, Hu KF, Qu JP, Kang YB. Org Lett, 2017, 19: 5593–5596
Marko JA, Durgham A, Bretz SL, Liu W. Chem Commun, 2019, 55: 937–940
Yang G, Zhang Q, Miao H, Tong X, Xu J. Org Lett, 2005, 7: 263–266
Dohi T, Takenaga N, Goto A, Fujioka H, Kita Y. J Org Chem, 2008, 73: 7365–7368
Telvekar VN, Sasane KA. Synth Commun, 2012, 42: 1325–1329
Meng L, Su J, Zha Z, Zhang L, Zhang Z, Wang Z. Chem Eur J, 2013, 19: 5542–5545
Zhang W, Gacs J, Arends IWCE, Hollmann F. ChemCatChem, 2017, 9: 3821–3826
Hruszkewycz DP, Miles KC, Thiel OR, Stahl SS. Chem Sci, 2017, 8: 1282–1287
Li J, Bao W, Tang Z, Guo B, Zhang S, Liu H, Huang S, Zhang Y, Rao Y. Green Chem, 2019, 21: 6073–6081
Liu KJ, Duan ZH, Zeng XL, Sun M, Tang Z, Jiang S, Cao Z, He WM. ACS Sustain Chem Eng, 2019, 7: 10293–10298
Zhu X, Liu Y, Liu C, Yang H, Fu H. Green Chem, 2020, 22: 4357–4363
Zhong PF, Lin HM, Wang LW, Mo ZY, Meng XJ, Tang HT, Pan YM. Green Chem, 2020, 22: 6334–6339
He MX, Mo ZY, Wang ZQ, Cheng SY, Xie RR, Tang HT, Pan YM. Org Lett, 2020, 22: 724–728
Meng X, Zhong P, Wang Y, Wang H, Tang H, Pan Y. Adv Synth Catal, 2020, 362: 506–511
Wang X, Zhong Y, Mo Z, Wu S, Xu Y, Tang H, Pan Y. Adv Synth Catal, 2021, 363: 208–214
Xu K, Zhang Z, Qian P, Zha Z, Wang Z. Chem Commun, 2015, 51: 11108–11111
Wang Y, Qian P, Su JH, Li Y, Bi M, Zha Z, Wang Z. Green Chem, 2017, 19: 4769–4773
Qian P, Zhou Z, Hu K, Wang J, Li Z, Zha Z, Wang Z. Org Lett, 2019, 21: 6403–6407
Hu K, Zhang Y, Zhou Z, Yang Y, Zha Z, Wang Z. Org Lett, 2020, 22: 5773–5777
Li Z, Sun Q, Qian P, Hu K, Zha Z, Wang Z. Chin Chem Lett, 2020, 31: 1855–1858
Zhang Z, Su J, Zha Z, Wang Z. Chem Commun, 2013, 49: 8982–8984
Zhang Z, Su J, Zha Z, Wang Z. Chem Eur J, 2013, 19: 17711–17714
Bégué JP, Delpon DB, Crousse B. Synlett, 2004, 1: 18–29
Shuklov I, Dubrovina N, Börner A. Synthesis, 2007, 2007(19): 2925–2943
Legault CY. CYLview 1.0b. Universit de Sherbrooke, 2009. http://www.cylview.org
Masui M, Hara S, Ueshima T, Kawaguchi T, Ozaki S. Chem Pharm Bull, 1983, 31: 4209–4211
Ueda C, Noyama M, Ohmori H, Masui M. Chem Pharm Bull, 1987, 35: 1372–1377
Horn EJ, Rosen BR, Chen Y, Tang J, Chen K, Eastgate MD, Baran PS. Nature, 2016, 533: 77–81
Kawamata Y, Yan M, Liu Z, Bao DH, Chen J, Starr JT, Baran PS. J Am Chem Soc, 2017, 139: 7448–7451
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21672200, 21772185) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000). We are grateful for the assistance of the product characterization from the National Demonstration Center for Experimental Chemistry Education of University of Science and Technology of China. The numerical calculations in this paper have been done on the supercomputing system in the Supercomputing Center of University of Science and Technology of China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, Z., Zhang, Y., Li, K. et al. Selective electrochemical oxidation of aromatic hydrocarbons and preparation of mono/multi-carbonyl compounds. Sci. China Chem. 64, 2134–2141 (2021). https://doi.org/10.1007/s11426-021-1061-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-1061-x