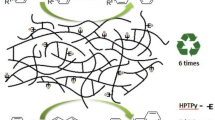
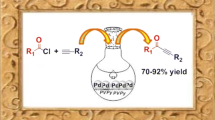
Abstract
Palladium nanoparticles immobilized on a cross-linked imidazolium-containing polymer were evaluated as a catalyst for Suzuki carbon-carbon cross-coupling reactions using water as the solvent. The nanocatalysts show good catalytic activities for aryl iodides and aryl bromides and moderate activity with aryl chloride substrates. Coupling of sterically hindered substrates could also be achieved in reasonable yields. The heterogeneous catalyst is stable, can be stored without precautions to exclude air or moisture, and can be easily recycled and reused.
Similar content being viewed by others
References
Miyaura N, Suzuki A. Chem Rev, 1995, 95: 2457–2483
Hassan J, Sevignon M, Gozzi C, Schulz E, Lemaire M. Chem Rev, 2002, 102: 1359–1469
Molander GA, Ellis N. Accounts Chem Res, 2007, 40: 275–286
Martin R, Buchwald SL. Accounts Chem Res, 2008, 41: 1461–1473
Littke AF, Dai CY, Fu GC. J Am Chem Soc, 2000, 122: 4020–4028
Arpad M. Chem Rev, 2011, 111: 2251–2320
Artok L, Bulut H. Tetrahedron Lett, 2004, 45: 3881–3884
Papp A, Galbacs G, Molnar R. Tetrahedron Lett, 2005, 46: 7725–7728
Iranpoor N, Firouzabadi H, Motevalli S, Talebi M. J Organomet Chem, 2012, 708: 118–124
Khalafi-Nezhad A, Panahi F. J Organomet Chem, 2012, 717: 141–146
Astruc D. Tetrahedron: Asymmetry, 2010, 21: 1041–1054
Deraedt C, Salmon L, Astruc D. Adv Synth Catal, 2014, 356: 2525–2538
Sobhani S, Pakdin-Parizi Z. Appl Catal A, 2014, 479: 112–120
Firouzabadi H, Iranpoor N, Gholinejad M, Akbari S, Jeddi N. Rsc Adv, 2014, 4: 17060–17070
Estrada GOD, Blanco ALP, da Silva JFM, Alonso CG, Fernandes-Machado NRC, Cardozo L, de Souza ROMA, Miranda LSME. Tetrahedron Lett, 2012, 53: 1089–1093
Du QW, Zhang W, Ma H, Zheng J, Zhou B, Li YQ. Tetrahedron, 2012, 68: 3577–3584
Gogoi A, Chutia SJ, Gogoi PK, Bora U. Appl Organomet Chem, 2014, 28: 839–844
McLachlan F, Mathews CJ, Smith PJ, Welton T. Organometallics, 2003, 22: 5350–5357
Wong HT, Pink CJ, Ferreira FC, Livingston AG. Green Chem, 2006, 8: 373–379
Liu Y, Wang SS, Liu W, Wan QX, Wu HH, Gao GH. Curr Org Chem, 2009, 13: 1322–1346
Chiappe C, Pieraccini D, Zhao DB, Fei ZF, Dyson PJ. Adv Synth Catal, 2006, 348: 68–74
Fei ZF, Zhao DB, Pieraccini D, Ang WH, Geldbach TJ, Scopelliti R, Chiappe C, Dyson PJ. Organometallics, 2007, 26: 1588–1598
Yang X, Fei ZF, Geldbach TJ, Phillips AD, Hartinger CG, Li YD, Dyson PJ. Organometallics, 2008, 27: 3971–3977
Zhao DB, Fei ZF, Geldbach TJ, Scopelliti R, Dyson PJ. J Am Chem Soc, 2004, 126: 15876–15882
Li CJ. Chem Rev, 2005, 105: 3095–3165
Lamblin M, Nassar-Hardy L, Hierso JC, Fouquet E, Felpin FX. Adv Synth Catal, 2010, 352: 33–79
Liu C, Zhang YX, Liu N, Qiu JS. Green Chem, 2012, 14: 2999–3003
Meric N, Aydemir M, Isik U, Ocak YS, Rafikova K, Pasa S, Kayan C, Durap F, Zazybin A, Temel H. Appl Organomet Chem, 2014, 28: 818–825
Wang Y, Liu JH, Xia CG. Tetrahedron Lett, 2011, 52: 1587–1591
Zhao DB, Fei ZF, Ang WH, Dyson PJ. Small, 2006, 2: 879–883
Zhang JC, Meng LJ, Zhao DB, Fei ZF, Lu QH, Dyson PJ. Langmuir, 2008, 24: 2699–2704
Yang X, Fei ZF, Zhao DB, Ang WH, Li YD, Dyson PJ. Inorg Chem, 2008, 47: 3292–3297
Fu CL, Meng LJ, Lu QH, Fei ZF, Dyson PJ. Adv Funct Mater, 2008, 18: 857–864
Ghazali-Esfahani S, Song HB, Paunescu E, Bobbink FD, Liu HZ, Fei ZF, Laurenczy G, Bagherzadeh M, Yan N, Dyson PJ. Green Chem, 2013, 15: 1584–1589
Jiao NM, Li ZL, Wang Y, Liu JH, Xia CG. Rsc Adv, 2015, 5: 26913–26922
Liu G, Hou MQ, Song JY, Jiang T, Fan HL, Zhang ZF, Han BX. Green Chem, 2010, 12: 65–69
Arras J, Paki E, Roth C, Radnik J, Lucas M, Claus P. J Phys Chem C, 2010, 114: 10520–10526
Parvulescu VI, Hardacre C. Chem Rev, 2007, 107: 2615–2665
Durand J, Teuma E, Malbosc F, Kihn Y, Gomez M. Catal Commun, 2008, 9: 273–275
Cui YG, Biondi I, Chaubey M, Yang X, Fei ZF, Scopelliti R, Hartinger CG, Li YD, Chiappe C, Dyson PJ. Phys Chem Chem Phys, 2010, 12: 1834–1841
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghazali-Esfahani, S., Păunescu, E., Bagherzadeh, M. et al. A simple catalyst for aqueous phase Suzuki reactions based on palladium nanoparticles immobilized on an ionic polymer. Sci. China Chem. 59, 482–486 (2016). https://doi.org/10.1007/s11426-015-5542-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-015-5542-3