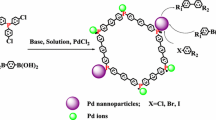
Abstract
An outstanding heterogeneous catalyst was successfully prepared by immobilization of palladium nanoparticles (Pd NPs) with polymer containing 4′‐(4‐hydroxyphenyl)‐2,2′:6′,2″‐terpyridine (HPTPy) ligand. The polymer cross‐linked with trimethylolpropane triacrylate (TMPTA) units was synthesized by polymerization of itaconic acid-HPTPy (ITC-HPTPy) monomer (so-called cross‐linked poly (ITC- HPTPy)). The cross‐linked poly (ITC-HPTPy) can stabilize the Pd NPs effectively against aggregation, thereby improving the catalytic efficiency of Pd NPs. The presence of Pd NPs on the polymer was confirmed by various physicochemical techniques. The resulting cross‐linked poly (ITC-HPTPy)-Pd was applied as a highly effective recyclable catalyst in Suzuki–Miyaura and Mizoroki–Heck coupling reactions under low Pd-loading conditions and straightforward methods, and provided the corresponding products with excellent yields (up to 98%), high catalytic activities (TOF up to 213 h−1). The catalyst can be separated from the reaction mixture by centrifugation and can be reused consecutive six times with slight reduction in catalytic activity.
Graphic Abstract
Pd NPs immobilized with polymer containing terpyridine ligand are highly active heterogeneous catalysts for Suzuki–Miyaura and Mizoroki–Heck coupling reactions.















Similar content being viewed by others
References
Fortman GC, Nolan SP (2011) N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: a perfect union. Chem Soc Rev 40:5151–5169
Corbet JP, Mignani G (2006) Selected patented cross-coupling reaction technologies. Chem Rev 106:2651–2710
Nicolaou KC, Bulger PG, Sarlah D (2005) Palladium-catalyzed cross-coupling reactions in total synthesis. Angew Chem Int Ed 44:4442–4489
Aijaz A, Xu Q (2014) Catalysis with metal nanoparticles immobilized within the pores of metal–organic frameworks. J Phys Chem Lett 5:1400–1411
Jiang Y, Gao Q (2006) Heterogeneous hydrogenation catalyses over recyclable Pd (0) nanoparticle catalysts stabilized by PAMAM-SBA-15 organic–inorganic hybrid composites. J Am Chem Soc 128:716–717
Ramirez E, Jansat S, Philippot K, Lecante P, Gomez M, Masdeu-Bultó AM, Chaudret B (2004) Influence of organic ligands on the stabilization of palladium nanoparticles. J Organomet Chem 689:4601–4610
Tatumi R, Akita T, Fujihara H (2006) Synthesis of small palladium nanoparticles stabilized by bisphosphine BINAP bearing an alkyl chain and their palladium nanoparticle-catalyzed carbon–carbon coupling reactions under room-temperature. Chem Commun 31:3349–3351
Sobhani S, Zeraatkar Z, Zarifi F (2015) Pd complex of an NNN pincer ligand supported on γ-Fe2O3@ SiO2 magnetic nanoparticles: a new catalyst for Heck, Suzuki and Sonogashira coupling reactions. New J Chem 39:7076–7085
Kwak Y, Matyjaszewski K (2009) ARGET ATRP of methyl methacrylate in the presence of nitrogen-based ligands as reducing agents. Polym Int 58:242–247
Targhan H, Hassanpour A, Bahrami K (2019) Highly efficient polymer-stabilized palladium heterogeneous catalyst: synthesis, characterization and application for Suzuki-Miyaura and Mizoroki-Heck coupling reactions. Appl Organomet Chem. https://doi.org/10.1002/aoc.5121
Pomogailo AD (2004) Catalysis by heterogenized metal polymers: advances and prospects. Kinet Catal 45:61–104
Kobayashi S, Nagayama SA (1996) polymer-supported scandium catalyst. J Org Chem 61:2256–2257
Zhao D, Ding K (2013) Recent advances in asymmetric catalysis in flow. ACS Catal 3:928–944
Puglisi A, Benaglia M, Chiroli V (2013) Stereoselective organic reactions promoted by immobilized chiral catalysts in continuous flow systems. Green Chem 15:1790–1813
Folarin OM, Sadiku ER, Maity A (2013) Polymer-noble metal nanocomposites. Int J Phys Sci 6:4869–4882
Spahni W, Calzagerri G (1984) Synthese von para-substituierten phenyl-terpyridin liganden. Helv Chim Acta 67:450–454
Bahrami K, Targhan H (2019) A new strategy to design a graphene oxide supported palladium complex as a new heterogeneous nanocatalyst and application in carbon–carbon and carbon-heteroatom cross-coupling reactions. Appl Organometal Chem. https://doi.org/10.1002/aoc.4842
Putta C, Sharavath V, Sarkar S, Ghosh S (2015) Palladium nanoparticles on β-cyclodextrin functionalised graphene nanosheets: a supramolecular based heterogeneous catalyst for C-C coupling reactions under green reaction conditions. RSC Adv 5:6652–6660
Villa A, Wang D, Spontoni P, Arrigo R, Su D, Prati L (2010) Nitrogen functionalized carbon nanostructures supported Pd and Au–Pd NPs as catalyst for alcohols oxidation. Catal Today 157:89–93
Hulubei C, Vlad CD, Stoica I, Popovici D, Lisa G, Nica SL, Barzic AI (2014) New polyimide-based porous crosslinked beads by suspension polymerization: physical and chemical factors affecting their morphology. J Polym Res 21:514
Shaik M, Ali Z, Khan M, Kuniyil M, Assal M, Alkhathlan H, Al-Warthan A, Siddiqui M, Khan M, Adil S (2017) Green synthesis and characterization of palladium nanoparticles using Origanum vulgare L. extract and their catalytic activity. Molecules 22:165–177
Gholinejad M, Hamed F, Biji P (2015) A novel polymer containing phosphorus–nitrogen ligands for stabilization of palladium nanoparticles: an efficient and recyclable catalyst for Suzuki and Sonogashira reactions in neat water. Dalton Trans 44:14293–14303
Long Y, Liu Y, Zhao Z, Long Y, Liu Y, Zhao Z, Luo S, Wu W, Wu L, Wen H, Wang RQ, Ma J (2017) Distinctive morphology effects of porous-spherical/yolk-shell/hollow Pd-nitrogen-doped-carbon spheres catalyst for catalytic reduction of 4-nitrophenol. J Colloid Interfaces Sci 496:465–473
Bahrami K, Khodamorady M (2018) Design of BNPs-TAPC palladium complex as a reusable heterogeneous nanocatalyst for the O-arylation of phenols and N-arylation of amines. Catal Lett 149:688–698
Labulo AH, Omondi B, Nyamori VO (2018) Suzuki-Miyaura reaction and solvent free oxidation of benzyl alcohol by Pd/nitrogen-doped CNTs catalyst. J Mater Sci 53:15817–15836
Long Y, Zhao Z, Wu L, Luo S, Wen H, Wu W, Zhang H, Ma J (2017) Distinctive ligand effects of functionalized magnetic microparticles immobilizing palladium acetate as heterogeneous coordination catalysts for selective oxidation of styrene to acetophenone. Mol Catal 433:291–300
Peng YY, Liu J, Lei X, Yin Z (2010) Room-temperature highly efficient Suzuki-Miyaura reactions in water in the presence of Stilbazo. Green Chem 12:1072–1075
Zeng X, Zhang T, Qin Y, Wei Z, Luo M (2009) Synthesis of a carbene transfer organometallic polymer and application to forming a recyclable heterogeneous catalyst for the Suzuki reactions of aryl chlorides. Dalton Trans 39:8341–8348
Hey DH, Orman S, Williams GH (1965) Homolytic aromatic substitution. Part XXXII. Some reactions with meta-substituted phenyl radicals. J Chem Soc. https://doi.org/10.1039/JR9650000101
Xu HJ, Zhao YQ, Zhou XF (2011) Palladium-catalyzed Heck reaction of aryl chlorides under mild conditions promoted by organic ionic bases. J Org Chem 76:8036–8041
Iranpoor N, Firouzabadi H, Tarassoli A, Fereidoonnezhad M (2010) 1, 3, 2, 4-Diazadiphosphetidines as new P-N ligands for palladium-catalyzed Heck reaction in water. Tetrahedron 66:2415–2421
Kim S, Cho HJ, Shin DS, Lee SM (2017) Recyclable and eco-friendly Pd-complexed graphene oxide/N-heterocyclic carbene catalyst for various coupling reactions in aqueous phase. Tetrahedron Lett 58:2421–2425
Dong D, Li Z, Liu D, Yu N, Zhao H, Chen H, Liu J, Liu D (2018) Postsynthetic modification of single Pd sites into uncoordinated polypyridine groups of a MOF as the highly efficient catalyst for Heck and Suzuki reactions. New J Chem 42:9317–9323
Khajehzadeh M, Moghadam M (2018) A new poly (N–heterocyclic carbene Pd complex) immobilized on nano silica: an efficient and reusable catalyst for Suzuki-Miyaura, Sonogashira and Heck-Mizoroki C-C coupling reactions. J Organomet Chem 863:60–69
Singh AS, Shelkar RS, Nagarkar JM (2015) Palladium (II) on functionalized NiFe2O4: an efficient and recyclable phosphine-free heterogeneous catalyst for Suzuki coupling reaction. Catal Lett 145:723–730
Bahrami K, Kamrani SN (2018) Synthesis, characterization and application of graphene palladium porphyrin as a nanocatalyst for the coupling reactions such as: suzuki-Miyaura and Mizoroki-Heck. Appl Organomet Chem 32:e4102
Kwon TH, Cho KY, Baek KY, Yoon HG, Kim BM (2017) Recyclable palladium–graphene nanocomposite catalysts containing ionic polymers: efficient Suzuki coupling reactions. RSC Adv 7:11684–11690
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Targhan, H., Hassanpour, A., Sohrabnezhad, S. et al. Palladium Nanoparticles Immobilized with Polymer Containing Nitrogen-Based Ligand: A Highly Efficient Catalyst for Suzuki–Miyaura and Mizoroki–Heck Coupling Reactions. Catal Lett 150, 660–673 (2020). https://doi.org/10.1007/s10562-019-02981-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02981-7