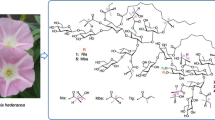
Abstract
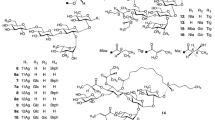
Ipomoea muricata (L.) Jacq. seeds (Convolvulaceae) are used as a traditional laxative and carminative medicine. Muricatins XIV (1), XV (2), XVI (3), and XVII (4), were isolated from I. muricata seeds as four new resin glycosides, along with seven known compounds, three of which were isolated for the first time as natural products; their structures were determined using MS and NMR spectroscopy. Compounds 1–4 are macrolactones (jalapins); the sugar moieties of 1, 2, and 4 are partially acylated with 2S-methylbutyric acid, while that of 3 is esterified with 2S-methylbutyric and 2S-methyl-3S-hydroxybutyric acids. In addition, the antiviral activities of the seven compounds obtained in this study, together with five known compounds obtained in our previous study into resin glycosides from I. muricata seeds, were evaluated against herpes simplex virus type 1 (HSV-1); their cytotoxicities against HL-60 human promyelocytic leukemia cells were also investigated. All examined jalapins exhibited similar or slightly weaker anti-HSV-1 activities than acyclovir, the positive control; however, the glycosidic acid of 4 was inactive, while its methyl ester was weakly active. On the other hand, cytotoxicity testing against HL-60 cells showed similar results to those observed during anti-HSV-1 activity testing, with the exception that one jalapin was less active.
Graphical Abstract




Similar content being viewed by others
References
Ono M (2017) Resin glycosides from Convolvulaceae plants. J Nat Med 71:591–604
Pereda-Miranda R, Rosas-Ramírez D, Castańeda-Gómez J (2010) Resin glycosides from morning glory family. In: Kinghorn AD, Falk H, Kobayashi J (eds) Progress in the chemistry of organic natural products, vol 92. Springer, New York, pp 77–153
Ono M, Yuhara N, Shimohara T, Matsubara S, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Zhou JR, Yoshimitsu H, Nohara T (2021) Calyhedins I–VI: resin glycosides from the rhizomes of Calystegia hederacea. Phytochemistry. https://doi.org/10.1016/j.phytochem.2021.112888
Ono M, Shimohara T, Yuhara N, Matsubara S, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Yoshimitsu H, Nohara T (2023) Four new resin glycosides, calyhedins VII–X, from the rhizomes of Calystegia hederacea. Nat Prod Res 37:1328–1337
Ono M, Taketomi S, Kakiki Y, Nishikawa H, Yauda S, Tsuchihashi R, Okawa M, Kinjo J, Miyashita H, Yoshimitsu H, Nohara T (2022) Two new resin glycosides, muricatins XII and XIII, from the seeds of Ipomoea muricata. Nat Prod Res. https://doi.org/10.1080/14786419.2022.2125970
Ono M, Yamano Y, Shimohara T, Yuhara N, Nodoca M, Nishikawa H, Yasuda S, Miyashita H, Yoshimitsu H, Tsuchihashi R, Okawa M, Kinjo J (2023) Five new resin glycosides, calyhedins XI–XV, from Calystegia hederacea. J Nat Med 77:774–791
Yoshikawa K, Yagi C, Hama H, Tanaka M, Arihara S, Hashimoto T (2010) Ipomotaosides A–D, resin glycosides from the aerial parts of Ipomoea batatas and their inhibitory activity against COX-1 and COX-2. J Nat Prod 73:1763–1766
Figueroa-González G, Jacobo-Herrera N, Zentella-Dehesa A, Pereda-Miranda R (2012) Reversal of multidrug resistance by morning glory resin glycosides in human breast cancer cells. J Nat Prod 75:93–97
Wang WQ, Liu SS, Song WB, Xuan LJ (2018) Resin glycosides from the seeds of Ipomoea muricata and their multidrug resistance reversal activities. Phytochemistry 149:24–30
Ono M, Takigawa A, Kanemaru Y, Kawakami G, Kabata K, Okawa M, Kinjo J, Yokomizo K, Yoshimitsu H, Nohara T (2014) Calysolins V-IX, resin glycosides from Calystegia soldanella and their antiviral activity toward herpes. Chem Pharm Bull 62:97–105
Ono M, Kawakami G, Takigawa A, Kabata K, Okawa M, Kinjo J, Yokomizo K, Yoshimitsu H, Nohara T (2014) Calysolins X–XIII, resin glycosides from Calystegia soldanella and their antiviral activity toward herpes simplex virus. Chem Pharm Bull 62:839–844
Ono M, Takigawa A, Muto H, Kabata K, Okawa M, Kinjo J, Yokomizo K, Yoshimitsu H, Nohara T (2015) Antiviral activity of four new resin glycosides calysolins XIV–XVII from Calystegia soldanella against herpes simplex virus. Chem Pharm Bull 63:641–648
Ono M, Kanemaru Y, Yasuda S, Okawa M, Kinjo J, Miyashita H, Yokomizo K, Yoshimitsu H, Nohara T (2017) A new resin glycoside from Calystegia soldanella and its antiviral activity towards herpes. Nat Prod Res 31:2660–2664
Khare CP (2007) Indian medical plants, an illustrated dictionary. Springer, Berlin, p 332
Noda N, Kobayashi H, Miyahara K, Kawasaki T (1988) Resin glycoside II. Identification and charcaterization of the component organic and glycosidic acids of the crude resin glycoside from the seeds of Ipomoea muricata. Chem Pharm Bull 36:627–633
Ono M, Yamada F, Noda N, Kawasaki T, Miyahara K (1993) Resin glycosides. XVIII. Determination by Mosher’s method of the absolute configurations of mono- and dihydroxyfatty acids originated from resin glycosides. Chem Pharm Bull 41:1023–1026
Noda N, Nishi M, Miyahara K, Kawasaki T (1988) Resin glycosides. IV. Two new resin glycosides, muricatins VII and VIII, from the seeds of Ipomoea muricata. Chem Pharm Bull 36:1707–1713
Noda N, Kobayashi H, Miyahara K, Kawasaki T (1988) Resin glycosides. III. Isolation and structural study of the genuine resin glycosides, muricatins I–VI, from the seeds of Ipomoea muricata. Chem Pharm Bull 36:920–929
Ono M, Taketomi S, Kakiki Y, Yauda S, Okawa M, Kinjo J, Miyashita H, Yoshimitsu H, Nohara T (2021) A new glycosidic acid, muricatic acid D, and resin glycosides, muricatins X and XI, from the crude resin glycoside fraction of the seeds of Ipomoea muricata. Chem Pharm Bull 69:291–297
Ono M, Taketomi S, Kakiki Y, Yauda S, Okawa M, Kinjo J, Yoshimitsu H, Nohara T (2016) A new resin glycoside, muricatin IX, from the seeds of Ipomoea muricata. Chem Pharm Bull 64:1408–1410
Uemura K, Kimura S, Saito Y, Koyama S, Nishikawa H, Yasuda S, Miyashita H, Yoshimitsu H, Tsuchihashi R, Okawa M, Kinjo J, Ono M (2022) Identification and characterization of organic and glycosidic acids in the crude resin glycoside fraction from the leaves and stems of Calystegia japonica. J Nat Med 77:284–297
Ono M, Ichihara Y, Saito N, Yamada M, Yuuki K, Nawata M, Tsutsumi S, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Miyashita H, Yoshimitsu H, Nohara T (2020) Identification and characterization of organic and glycosidic acids in crude resin glycoside fraction from Calystegia hederacea. J Nat Med 74:200–211
Schinazi RF, Peres J, Williams CC, Chance D, Nahmias A (1982) Effect of combinations of acyclovir with vidarabine or its 5′-monophospate on herpes simplex viruses in cell culture and in mice. Antimicrob Agents Chemother 22:499–507
Acknowledgements
We express our appreciation to Mr. H. Harazono of Fukuoka University for the measurement of the FAB-MS and ESI-TOF-MS. This research was partially supported by the Research and Study Project of Tokai University General Research Organization (Kanagawa, Japan).
Funding
This research was partially supported by a Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI Grant Numbers JP16K08306 and JP20K07117).
Author information
Authors and Affiliations
Contributions
Prof. Dr. Ono supervised the research, wrote the manuscript, and analyzed the data. Mr. D. Tenmaya, M. Tarumi, S. Satou, and K. Tsuji purified the compounds. Mr. Nishikawa and Prof. Dr. Yasuda were responsible for the antitumor activity. Prof. Dr. K. Yokomizo and Dr. J. Zhou were responsible for the anti-HSV-1 assay. Dr. Miyashita, Prof. Dr. Yoshimitsu, Dr. Tsuchihasi, Prof. Dr. Okawa, and Prof. Dr. Kinjo performed instrumental analysis and data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ono, M., Tenmaya, D., Tarumi, M. et al. Four new resin glycosides from Ipomoea muricata seeds: muricatins XIV–XVII. J Nat Med (2024). https://doi.org/10.1007/s11418-024-01787-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11418-024-01787-1