Abstract
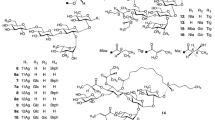
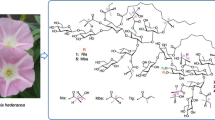
The alkaline hydrolysis of the crude resin glycoside fraction from the leaves and stems of the plant Calystegia japonica Choisy (Convolvulaceae) yielded organic acid and glycosidic acid fractions. The organic acid fraction was esterified with p-bromophenacyl bromide to obtain p-bromophenacyl 2R-methyl-3R-hydroxybutyrate (1) and p-bromophenacyl (E)-2-methylbut-2-enoate (2). By treating the glycosidic acid fraction with trimethylsilyldiazomethane–hexane, seven new methyl esters of glycosidic acids, namely calyjaponic acid A methyl ester (3) calyjaponic acid B methyl ester (5), calyjaponic acid C methyl ester (6), calyjaponic acid D methyl ester (7), calyjaponic acid E methyl ester (8), calyjaponic acid F methyl ester (9), and calyjaponic acid G methyl ester (10), were isolated along with one known ester (4). Their structures were characterized based on spectroscopic and chemical analyses. Compounds 3–8 had the same sugar moiety, α-L-rhamnopyranosyl-(1 → 2)-O-β-D-glucopyranosyl-(1 → 2)-[O-α-L-rhamnopyranosyl-(1 → 6)]-O-β-D-glucopyranose, and the aglycones of 3–8 were methyl 3S,11S-dihydroxyhexadecanoate, methyl 3S,12S-dihydroxyhexadecanoate, methyl 11S-hydroxyhexadecanoate, methyl 11S-hydroxypentadecanoate, methyl 3S,11S-dihydroxypentadecanoate, and methyl 3S,12S-dihydroxypentadecanoate, respectively. Compounds 9 and 10 were derivatives of 3 and 4, respectively, in which the C-6 of the second glucosyl residue was methylated. Compounds 6–8 contained methyl esters of unusual odd-carbon fatty acids as aglycones. The cytotoxicity of the crude resin glycoside fraction and 3 against HL-60 human promyelocytic leukemia cells was evaluated further; both were either weakly active or inactive compared to the positive control, cisplatin.
Graphical abstract



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Ono M (2017) Resin glycosides from Convolvulaceae plants. J Nat Med 71:591–604
Pereda-Miranda R, Rosas-Ramírez D, Castańeda-Gómez J (2010) Resin glycosides from morning glory family. In: Kinghorn AD, Falk H, Kobayashi J (eds) Progress in the chemistry of organic natural products, vol 92. Springer-Verlag, New York, pp 77–153
Ono M, Yuhara N, Shimohara T, Matsubara S, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Zhou JR, Yoshimitsu H, Nohara T (2021) Calyhedins I-VI: resin glycosides from the rhizomes of Calystegia hederacea. Phytochemistry. https://doi.org/10.1016/j.phytochem.2021.112888
Ono M, Shimohara T, Yuhara N, Matsubara S, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Yoshimitsu H, Nohara T (2021) Four new resin glycosides, calyhedins VII–X, from the rhizomes of Calystegia hederacea. Nat Prod Res. https://doi.org/10.1080/14786419.2021.2005593
Yoshikawa K, Yagi C, Hama H, Tanaka M, Arihara S, Hashimoto T (2010) Ipomotaosides A-D, resin glycosides from the aerial parts of Ipomoea batatas and their inhibitory activity against COX-1 and COX-2. J Nat Prod 73:1763–1766
Figueroa-González G, Jacobo-Herrera N, Zentella-Dehesa A, Pereda-Miranda R (2012) Reversal of multidrug resistance by morning glory resin glycosides in human breast cancer cells. J Nat Prod 75:93–97
Ono M, Takigawa A, Kanemaru Y, Kawakami G, Kabata K, Okawa M, Kinjo J, Yokomizo K, Yoshimitsu H, Nohara T (2014) Calysolins V-IX, resin glycosides from Calystegia soldanella and their antiviral activity toward herpes. Chem Pharm Bull 62:97–105
Hotta M, Ogata K, Nitta A, Hosikawa K, Yanagi M, Yamazaki K (eds)(1989) Useful plants of the world. Heibonsha Ltd., Tokyo, p 197
Takagi S, Yamaki M, Masuda K, Kubota M (1977) Studies on the purgative drugs. IV. On the constituents of Calystegia japonica Choisy. Yakugaku Zasshi 97:1369–1371
Ahn N-R, Ko J-M, Cha H-C (2012) Comparison of flavonoid profiles between leaves and stems of Calysegia soldanella and Calystegia japonica. Am J Plant Sci 3:1073–1076
Murai Y, Setoguchi H, Ono E, Iwashina T (2015) Flavonoids and their qualitative variation in Calystegia soldanella. Nat Prod Commun 10:429–432
Ono M, Taketomi S, Nishikawa H, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Miyashita H, Yoshimitsu H, Nohara T (2022) Two new resin glycosides, muricatins XII and XIII, from the seeds of Ipomoea muricata. Nat Prod Res. https://doi.org/10.1080/14786419.2022.2125970
Ono M, Ichihara Y, Saito N, Yamada M, Yuuki K, Nawata M, Tsutsumi S, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Miyashita H, Yoshimitsu H, Nohara T (2020) Identification and characterization of organic and glycosidic acids in crude resin glycoside fraction from Calystegia hederacea. J Nat Med 74:200–211
Ono M, Taketomi S, Kakiki Y, Yasuda S, Okawa M, Kinjo J, Miyashita H, Yoshimitsu H, Nohara T (2021) A new glycosidic acid, muricatic acid D, and resin glycosides, muricatins X and XI, from the crude resin glycoside fraction of the seeds of Ipomoea muricata. Chem Pharm Bull 69:291–297
Ono M, Noda N, Kawasaki T, Miyahara K (1990) Resin glycosides. VII. Reinvestigation of the component organic and glycosidic acids of pharbitin, the crude ether-insoluble resin glycoside (“convolvulin”) of pharbitidis semen (seeds of Pharbitis nil). Chem Pharm Bull 38:1892–1897
Ono M, Yamada F, Noda N, Kawasaki T, Miyahara K (1993) Resin glycosides. XVIII. Determination by Mosher’s method of the absolute configurations of mono- and dihydroxyfatty acids originated from resin glycosides. Chem Pharm Bull 41:1023–1026
Tanaka T, Nakashima T, Ueda T, Tomii K, Kouno I (2007) Facile discrimination of aldose enantiomers by reversed-pase HPLC. Chem Pharm Bull 55:899–901
Seo S, Tomita Y, Tori K, Yoshimura Y (1978) Determination of the absolute configuration of a secondary hydroxy group in a chiral secondary alcohol using glycosidation shifts in carbon-13 nuclear magnetic resonance spectroscopy. J Am Chem Soc 100:3331–3339
Kasai R, Suzuo M, Asakawa J, Tanaka O (1977) Carbon-13 chemical shifts of isoprenoid-β-D-glucopyranosides and -β-D-mannopyranosides. Stereochemical influences of aglycone alcohols. Tetrahedron Lett 2:175–178
Tori K, Seo S, Yoshimura Y, Arita H, Tomita Y (1977) Glycosidation shifts in carbon-13 NMR spectroscopy: carbon-13 signal shifts from aglycone and glucose to glucoside. Tetrahedron Lett 2:179–182
Ono M, Nishioka H, Fukushima T, Kunimatu H, Mine A, Kubo H, Miyahara K (2009) Components of Ether-Insoluble Resin Glycoside (Rhamonvolvulin) from Rhizoma Jalapae Braziliensis. Chem Pharm Bull 57:262–268
Ono M, Honda F, Karahashi A, Kawasaki T, Miyahara K (1997) Resin glycosides. XXV. Multifidins I and II, new jalapins, from the seed of Quamoclit × multifida. Chem Pharm Bull 45:1955–1960
Devies LA, Adams R (1928) Structures of convolvulinolic and jalapinolic acids. Synthesis of 11-hydroxypentadecanoic acid and 11-hydroxyhexadecanoic acids. J Am Chem Soc 50:1749–1755
Wagner H, Wenzel G, Chari VM (1978) The turpethinic acids of Ipomoea turpethum L. Plant Med 33:141–151
Dale JA, Mosher HS (1973) Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmanndelate, and α-methoxy-α-trifluoromethylphenylacetate (MTPA) esters. J Am Chem Soc 95:512–519
Çaliş Í, Sezgin Y, Döedi P, Tasdemir D (2007) Crypthophillic acids A, B, and C: Resin glycosides from aerial parts of Scrophularia crpthophila. J Nat Prod 70:43–47
Ono M, Fukunaga T, Kawasaki T, Miyahara K (1990) Resin Glycosides. VIII. Four new glycosidic acids, operculinic acids D, E, F and G, of the ether-soluble crude resin jlycosides (“Jalapin”) from Rhizoma Jalapae Braziliensis (roots of Ipomoea operculata). Chem Pharm Bull 38:2650–2655
Ding W, Jiang Z-H, Wu P, Xu L, Wei X (2012) Resin glycosides from the aerial parts of Operculina trupethum. Phytochemistry 81:165–174
Fan B-F, Lu YH, He Y, Li J-L, Chen G-T (2018) Arvensic acids A-D, novel heptasaccharide glycosidic acids as the alkaline hydrolysis products of crude resin glycosides from Convolvulus arvensis. Fitoterapia 131:209–214
Takigawa A, Setoguchi H, Okawa M, Kinjo J, Miyashita H, Yokomizo K, Yoshimitsu H, Nohara T, Ono M (2011) Identification and characterization of component organic and glycosidic acids of crude resin glycoside fraction from Calystegia soldanella. Chem Pharm Bull 59:1163–1168
Ono M, Saito N, Minamishima H, Yasuda S, Tsuchihashi R, Okawa M, Kinjo J, Miyashita H, Yoshimitsu H, Nohara T (2022) Two new glycosidic acids, calyhedic acids E and F, in crude resin glycoside fraction from Calystegia hederacea. Nat Prod Res 36:46–53
Ono M, Azuchi M, Ichio M, Jiyoubi Y, Tsutsumi S, Yasuda S, Tsuchihasi R, Okawa M, Kinjo J, Yoshimitsu H, Nohara T (2019) Seven new resin glycosides from the seeds of Quamoclit × multifida. J Nat Med 73:11–22
Ono M, Takigawa A, Mineno T, Yoshimitsu H, Nohara T, Ikeda T, Fukuda-Teramachi E, Noda N, Miyahara K (2010) Acylated glycosides of hydroxy fatty acid methyl esters generated from the crude resin glycoside (pharbitin) of seeds of Pharbitis nil by treatment with indium(III) chloride in methanol. J Nat Prod 73:1846–1852
Acknowledgements
We express our appreciation to Mr. H. Harazono of Fukuoka University for the measurement of the MS. This research was supported in part by a Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI Grant Numbers JP16K08306 and JP20K07117) and by the Research and Study Program/Project of Tokai University Educational System General Research Organization (Kanagawa, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict Of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uemura, K., Kimura, S., Saito, Y. et al. Identification and characterization of organic and glycosidic acids in the crude resin glycoside fraction from the leaves and stems of Calystegia japonica. J Nat Med 77, 284–297 (2023). https://doi.org/10.1007/s11418-022-01669-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-022-01669-4