Abstract
5-Nor stemmadenine alkaloids, isolated from the genus Tabernaemontana, display a range of bioactivity. 16-Hydroxy-16,22-dihydroapparicine, the active component of an extract from the Tabernaemontana sp. (dichotoma, elegans, and divaricate), exhibited potent antimalarial activity, representing the first such report of the antimalarial property of 5-nor stemmadenine alkaloids. We, therefore, decided to attempt the total synthesis of the compound to explore its antimalarial activity and investigate structure and bioactivity relationships. As a result, we completed the first total synthesis of 16-hydroxy-16,22-dihydroapparicine, by combining a phosphine-mediated cascade reaction, diastereoselective nucleophilic addition of 2-acylindole or methylketone via a Felkin–Anh transition state, and chirality transferring intramolecular Michael addition. We also clarified the absolute stereochemistries of the compound. Furthermore, we evaluated the activity of the synthetic compound, as well as that of some intermediates, all of which showed weak activity against chloroquine-resistant Plasmodium falciparum (K1 strain) malaria parasites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Naturally occurring chemicals represent a treasure trove of compounds which hold promise as the seeds of discovery for drugs and medicines and which may facilitate the elucidation of structure and function investigations of bioactivity [1]. Ōmura’s research group at the Kitasato Institute is a global pioneer in the search for bioactive agents that may be of use in developing drugs and medicines to fight to infection and combat tropical diseases (such as the filariases, malaria, trypanosomiasis, etc.), all originating from microbial metabolites. At present, 483 new compounds have been discovered, 26 of which have become useful, widely used agents in human and animal health, including the ground-breaking avermectins [2].
Malaria is one of the world’s worst health and socioeconomic problems, causing widespread death, disease, disability, and economic loss. Infection arises when a protozoal parasite of the Plasmodium genus is transmitted to humans via the bites of blood-feeding mosquitoes. Plasmodium falciparum parasites cause the most deadly form of the disease, which can cause death in a few days, especially if cerebral malaria develops. Generally, most deaths occur in children under 5 years old, although deaths have been reduced markedly by recent global initiatives to tackle the disease [3–5]. Commonly used drugs to combat malaria include quinine, chloroquine, mefloquine, halofantrine, and sulfadoxine/pyrimethamine (Fig. 1). However, drug resistance in parasites has usually developed quickly, rendering many of these drugs useless, preventing effective treatment and hindering disease elimination efforts. In 1972, Professor Tu Youyou discovered artemisinin to be the active ingredient in the plant Artemisia annua, which was commonly used in China to treat fever. Artemisinin derivatives became the most effective therapeutic drugs against malaria [6]. The World Health Organization (WHO) recommends artemisinin-based combination therapies (ACTs) for malaria treatment [7], a multidrug approach requiring the use of artemisinin together with other drugs to help offset the pace of drug resistance to artemisinin developing and spreading. ACTs are already compromised because the safety of artemisinin with regard to use during first trimester pregnancy is yet to be established and, worse, resistance to artemisinin derivatives developed almost immediately in locations along the Thai–Cambodian border [8–11]. Therefore, inexpensive and potent antimalarial drugs, especially those that have different modes of action, are urgently required on a probably continuing basis due to the ability of the malaria parasites to quickly develop drug resistance.
Many of the therapies currently in development use known antimalarial pharmacophores (e.g., aminoquinolines and/or peroxides), which have been chemically modified to overcome the failures of their predecessors [12]. Although these compounds have been important in the treatment of malaria, it would be highly advantageous to discover chemotypes with novel action mechanisms [13]. However, despite important advances in our understanding of the Plasmodium genome, the identification and validation of new drug targets have been challenging [14–16].
16-Hydroxy-16,22-dihydroapparicine (1), a known 5-nor stemmadenine alkaloid, was identified at the Kitasato Institute as a main component of a leaf’s MeOH extract from the plant Tabernaemontana dichotoma, which displayed antimalarial properties. The potent antimalarial activity of the complex leaf extract against chloroquine-resistant Plasmodium falciparum (K1 strain) parasites in vitro, and its moderate selectivity (against MRC-5 strain human cells) are summarized in Table 1. Natural compound 1 was originally isolated from a leaf of Tabernaemontana dichotoma in 1984 by the Verpoorte group [17] (Fig. 2). The relative structural determination of 1 was based on detailed NMR study, yet the absolute stereochemistry was not determined. As 1 has the potential to contain antimalarial activity, we decided to attempt the total synthesis of 1 to confirm its stereochemistry and investigate its antimalarial effect.
In this review, the total synthesis, stereochemical determination, and antimalarial activity of 16-hydroxy-16,22-dihydroapparicine are discussed [18, 19].
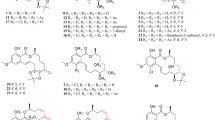
Naturally occurring compound 1 has the same framework as Apparicine (2), the first 5-nor stemmadenine alkaloid discovered, which was isolated from Aspidosperma dasycarpon more than 45 years ago [20, 21] (Fig. 3). There are currently 22 known 5-nor stemmadenine alkaloid compounds [22–32], with the compounds exhibiting a wide range of biological activity, including being antimicrobial [33–35] and antibacterial (antituberculoid) [32], as well as displaying opioid properties [36]. Consequently, these alkaloids are of considerable interest. The main structural feature of the alkaloids is the strained 1-azabicyclo[4.2.2]decane skeleton, including a single carbon connection, between the indole 3-position and aliphatic nitrogen moiety, which is a defining characteristic of these compounds. The relative stereochemistry of 2–5 has also been reported for conolidine (6), the completed asymmetric total synthesis being accomplished by Micalizio’s group [37].
Proposed biosynthesis
The special architecture involved, embodying a 1-azabicyclo[4.2.2]decane, is probably the result of the C-5 tryptamine atom being excised from the alkaloid stemmadenine by a retro-Mannich reaction. Some in vitro transformations of stemmadenine-type to 5-nor stemmadenine-type alkaloids have provided further support for this biogenetic model, which the following summarizes.
Kutney and co-workers reported the biosynthesis of the 1-azabicyclo[4.2.2]decane structure in the 5-nor stemmadenine alkaloids 50 years ago, using incorporated radioisotope experiments on the plant Aspidosperma pyricollum. Later, Lim and co-workers [38] reported partial synthesis of the pseudo-aminal type indole alkaloids, such as apparicine (2), using Potier’s expected biomimetic oxidative transformation from pericine (7) (Scheme 1).
Synthesis studies
Due to their unique structure and potentially useful biological activity, the total synthesis of 5-nor stemmadenine alkaloids has been reported by Bennasar et al. [39, 40], Micalizio [37], and Takayama [41] (Scheme 2). In addition, synthetic work on the 1-azabicyclo[4.2.2]decane skeleton core has been published by Joule [42, 43] and Weinreb’s group [44] (Scheme 3). A recent report of the total synthesis of (±)-apparicine (2) by Bennasar and co-workers [39, 40] detailed an approach which utilized an intramolecular Heck reaction. Micalizio and Takayama [37, 41] reported the total syntheses of conolidine (6), which could be derived from an iminium ion under intramolecular Mannich reaction. In addition, Micalizio and co-workers [37] clarified the absolute stereochemistry of 6.
In 1977, Joule and co-workers [42, 43] reported the synthesis of apparicine, detailing an approach to 2 which utilizes an intramolecular Mannich cyclization to construct the 1-azabicyclo[4.2.2]decane skeleton. Weinreb’s group [44] reported the construction of a 4-cyclic compound 17 using nitrosoalkene and indole in 2014.
Our synthetic approach used a distinctive reaction based on the hypothesis that the main structural feature of these alkaloids is the strained 1-azabicyclo[4.2.2]decane skeleton, including a single carbon connection between the indole 3-position and aliphatic nitrogen moiety, which is a gramine-type (or vinamidine-type) moiety (Fig. 4). This structure has a “push–pull” nature, which is stabilized by electron-donating or electron-withdrawing groups. For example, the aliphatic carbon–nitrogen bond of the gramine type (or vinamidine type) is easily cleaved by retro-Mannich reaction under acid [45], base [46–48], and thermal [49] conditions, and with various reagents (e.g., trialkylphosphine [50–55], Lewis acid [56], phthalimide [57], thiol [58, 59], and activated ester [60, 61]) to generate the indolinium cation. We, therefore, anticipated that the propendiamine moiety was an indicator of reactivity similar to the aminal, leading us to suppose the framework as a “pseudo-aminal type structure”.
To complete the total synthesis of (15S*,16S*)-16-hydroxy-16,22-dihydroapparicine (1), we designed a novel phosphineimine-mediated cascade reaction, without any isolated unstable intermediate (Scheme 4). The cascade reaction sequence was: (1) Staudinger reaction of an azide 21 with triphenylphosphine to generate phosphineimine intermediate 20 [62]; (2) intramolecular N-allylation of phosphineimine transformed into aminophosphinium 19 [63–65]; (3) aza-Wittig reaction of 19 with formaldehyde; and (4) intramolecular Mannich reaction; nucleophilic attack might be performed from the indole 3-position to iminium cation 18. We needed to solve two challenging issues. Firstly, the N-allylation of the phosphineimine group; phosphineimine has relatively high nucleophilicity, while the leaving group involves sufficient electrophilicity. Secondly, the formation of iminium cation using the aminophosphonium salt; there was no reported generation of iminium cation using the aminophosphonium salt and aldehyde via the aza-Wittig reaction. We found a solitary instance of the aminophosphonium salt with excess DMF to generating formamidinium salt [66]. However, the potential reactivity of the aminophosphonium salt has never been investigated. If we could overcome these challenges, an aminophosphonium salt (such as 19) could become a useful reactant for the aza-Wittig reaction. The key precursor 21 could be prepared from diastereoselective methylation of 2-acylindole 22 with completion of the C-16 stereochemistry outcome of the Felkin–Anh transition state [67–71] (Scheme 5). Compound 22 could be constructed with the indole nucleophile and azidoaldehyde 23.
To construct the C-15 stereocenter, we envisaged a remote stereocontrolled Michael reaction [72] of the α,β-unsaturated carboxamide 25 with the crotonic acid derivative.
Synthesis of the azidoaldehyde 23 began from commercially available cis-butenediol, to afford (−)-25 [73] (Scheme 6). With the Michael accepter in hand, we attempted the remote stereocontrolled Michael reaction of (−)-25, with only minor success, (−)-25 appearing with no stereoselection and in low yield, along with γ-adduct as an undesired product. Subsequently, olefin isomerization afforded the unsaturated E-olefin 24 as a 1:1 diastereomixture (at C-15). Then, eight steps functionalization provided the azidoaldehyde (±)-23 in 38 % overall yield.
With the azidoaldehyde (±)-23 and the N-phenylsulfonyl indole 27 [74–81] in hand, we examined the nucleophilic addition, the hydroxyindole (±)-28 being provided in 85 % yield as a single diastereomer (Scheme 7). Following the oxidation of (±)-28 to obtain the (±)-ketoindole, the N-phenylsulfonyl and pivaloyl groups were subsequently removed under basic solvolysis to provide the hydroxyketoindole (±)-22 in 87 % yield. Diastereoselective methylation of (±)-22 converted it to dihydroxyindole (±)-29 as a single diastereomer in excellent yield. The planar structure of (±)-29 was confirmed by HMQC and HMBC studies. We expected the stereoselectivity outcome to be the Felkin–Anh transition state and so sought a suitable leaving group on the allyl alcohol. We eventually discovered a 3-nitropyridyl group [82, 83] as an efficient leaving group, allowing conversion of the 3-nitropyridinylation of (±)-29 into the cascade reaction precursor (±)-21 in 93 % yield, using the process reported by Ballesteros and co-workers [84, 85]. We then attempted construction of the 1-azabicyclo[4.2.2]decane skeleton, including the pseudo-aminal moiety. The cascade reaction precursor (±)-21, with PPh3 at 60 °C, generated iminophosphorane, the reaction mixture subsequently being acidified using AcOH for activation of the 3-nitropyridyl group. Finally, formaldehyde and PPTS were added to the reaction mixture to convert the iminophosphonium cation, followed by a Mannich reaction to furnish (±)-1 in 88 % yield. The relative stereochemistry was confirmed by ROESY correlations (Fig. 5).
Structure determination
However, the spectral data of synthetic (±)-1 did not agree with that of naturally occurring 1 [17]. In particular, analysis of synthetic (±)-1, showed a ROESY relationship between H-18 or H-19 and 16-Me. Consequently, the relative stereochemistry of synthetic (±)-1 was determined to be a 15S*,16S*-configuration. Data of synthetic (±)-(15S*,16S*)-1 were then compared with naturally occurring compound (Table 2), with 1H and 13C NMR indicating differences of chemical shift (differences of all positions are shown in the experiment section). In 1H NMR, 16-Me and H-6α,β signals were registered more than 0.20 ppm and, furthermore, the 13C signals of the piperidine ring were greatly shifted from those seen in natural occurring 1. Therefore, we expected that the 16-Me group in naturally occurring 1 was on the opposite face for the tri-substituted exo-cyclic olefin. Accordingly, the relative stereochemistry was anticipated to be the 15S*,16R*-configuration.
To confirm this consideration, we set about the synthesis of 15S*,16R*-isomer. The disputed stereocenter was prepared from ketoindole and methyl anion via the Felkin–Anh transition state. Therefore, the R-configuration could be constructed with methylketone (±)-30 and indole nucleophile. We search and optimized nucleophilic addition using indole nucleophile. As a result, we found (t-butyldimethylsilyloxy)methyl (TBSOM) group [86–91] protected iodoindole as a suitable compound (Scheme 8). Hence, nucleophilic addition of 31 with (±)-30 was converted into (±)-32 in 97 % yield as a single diastereomer. The planar structure of (±)-32 was confirmed by 2D NMR study. Subsequently, global deprotection of (±)-32 obtained (±)-33 in excellent yields. Following the same reaction sequence as the synthesis of (±)-(15S*,16S*)-1 produced (±)-(15S*,16R*)-1. Characterization data provided for synthetic (±)-(15S*,16R*)-1 were fully consistent with the data for the naturally occurring compound reported by Verpoorte and co-workers [17] (Table 3). In addition, an NOE relationship was observed between H-14a and H-22 (i.e., 16-Me) (Fig. 6).
To clarify the cascade reaction mechanism, we attempted the experiment outlined in Scheme 9. At first, to provide the corresponding primary amine, a Staudinger reaction of (±)-34 with PPh3 was carried out under reflux condition to obtain the piperidine-indole (±)-37, without acidic activation of the 3-nitropyridinyl group. ESI mass-monitoring of the first reaction allowed phosphineimine 35 to be easily generated from (±)-34 and PPh3 without transformation into primary amine via solvolysis. In a time-dependent change, phosphineimine smoothly converted to the aminophosphonium cation 36. Though the 3-nitropyridinyl group was a low electrophile, it was unnecessary for acidic activation. We inferred that the 1,3-allylic strain [92] was a key component, occurring via the tri-substituted olefin. Therefore, the 3-nitropyridinyl group was located within close proximity of the phosphineimine group. Subsequent intramolecular Mannich reaction of piperidine-indole (±)-37 provided (±)-(15S*,16R*)-1 in 43 % yield, using formaldehyde and PPTS. We subsequently expected that the aza-Wittig reaction of 36 with formaldehyde could assist in generating the iminium cation precursor 38 in a cascade reaction.
Asymmetric total synthesis of 16-hydroxy-16,22-dihydroapparicine
We achieved the total synthesis of racemic 16-hydroxy-16,22-dihydroapparicine (1) and determined the true relative stereochemistry of the naturally occurring compound. In the next stage, we established the absolute stereochemistry of 1. In order to accomplish asymmetric total synthesis, we used the chiral methylketone 30 (Scheme 10), which could be supplied from azidobutyrolactone 39, including the appropriate functional groups. If 39 formed acetylbutyrolactone 40, its acetyl and ester moiety could be transformed into E-ethylidene and azido groups, respectively. Acetylbutyrolactone 40 was, therefore, our key intermediate, with synthetic manners for related compounds having already been reported by Smith’s group and others [93–96]. We expected that 40 would involve a C-15 stereocenter being constructed by the intramolecular chirality transferring Michael reaction. We expected to perform via 5-exo-cyclization in the ketoester 41, which should be stereo-specifically constructed by the Baldwin rule [97] and Thorpe–Ingold effect [98, 99].
Synthesis of the optically pure tri-substituted 40 began from commercially available (−)-(R)-methyl lactate 42, which, after with four steps of preparation, provided the ketoester (+)-41 in excellent yield (Scheme 11). With the optically pure (+)-41 in hand, we attempted the intramolecular chirality transferring Michael reaction [100–104]. Through extensive optimization, we found a suitable condition to provide (+)-40 in 91 % yield as a single diastereomer, and assignment of the relative stereochemistry was derived from the coupling constants and NOE correlation between α and γ protons. The key factor of the intramolecular chirality transferring Michael reaction was the solvent’s effect; polar solvent was stabilized to the anticipated transition state. The acetyl group of (+)-40 converted into the ethylidene moiety along with the separable Z-isomer. The tri-substituted olefin moiety was determined to be of E-configuration by NOE correlation. The configuration of the C-3 stereocenter of (−)-43 was determined after simple modification; hydrogenation of (−)-43 obtained a single diastereomer, and the stereochemistry was confirmed to be S-configuration by NOE and ROESY correlation. Compound (−)-43 was transformed into primary alcohol 44 by stepwise preparation; at first, selective hydrolysis of the ethyl ester group under basic condition generated carboxylic acid, followed by the corresponding acid anhydride. The furnished carboxylic anhydride was immediately reduced to the desired (−)-44 in 82 % yield over the two steps [105, 106]. Subsequently, four steps functionalization provided the chiral methylketone (+)-30 in excellent yield without racemization. The optical purity of the (+)-30 (97 %ee) was confirmed by chiral HPLC analysis. The R-isomer of (−)-30 was prepared in the same asymmetric synthetic manner from (−)-(S)-methyl lactate.
Finally, (+)-32 was exposed to the same procedure using (±)-(15S*,16R*)-16-hydroxy-16,22-dihydroapparicine 1 (Scheme 12). The cascade reaction precursor (−)-36 underwent the same cascade reaction condition as that for the synthesis of (±)-(15S*,16S*)-1, (±)-(15S*,16R*)-1 to give (+)-(15S,16R)-1. Characterization data proved that synthetic (+)-(15S,16R)-1 was fully consistent with the data for the natural compound, as reported by Verpoorte and co-workers [17]. The optical rotation of synthetic (+)-(15S,16R)-1, [α] 26D +112.2 (c 0.9, EtOH), compared well with the values reported for the natural sample, [α] 20D +129 (c 0.1, EtOH), and the optical rotation of synthetic (−)-(15R,16S)-1, [α] 26D −104.2 (c 0.1, EtOH), was prepared in an asymmetric synthetic manner. In addition, an NOE relationship was observed between H-14a and H-22 (i.e., 16-Me). Therefore, the C-16 stereochemistry was determined to be the R-configuration.
Biological activity
Naturally occurring and synthetic compounds were tested for antimalarial activity against Plasmodium falciparum parasites (chloroquine-resistant K1 strain and chloroquine-susceptible FCR3 strain) and for cytotoxicity (against human MCR-5 cells) [107–109], in comparison with the first-line antimalarial artemisinin.
The in vitro antimalarial activities and cytotoxicities of the naturally occurring and synthetic compounds are summarized in Table 1. As shown in Table 4, Tabernaemontana leaf extract (which includes (+)-(15S,16R)-16-hydroxy-16,22-dihydroapparicine) showed activity against both the chloroquine-resistant K1 strain and the chloroquine-sensitive FCR3 strains of Plasmodium falciparum (approximately 78-fold less potent than artemisinin, and with synthetic (±)-(15S*,16S*)-1 having no measurable impact on chloroquine-susceptible parasites). Synthetic (±)-(15S*,16S*)-1, (+)-(15S,16R)-1, (−)-1 displayed moderate to weak antimalarial activity (in the range of 9.0 to >12.5 μg/mL), while synthetic (−)-1 and intermediaries showed minimal impact (7.17 to >12.5 μg/mL). The cytotoxicities against human cells of all synthetic compounds were weak (IC50 of 17–75 μg/mL), on average similar to that of artemisinin.

The IC50 value of synthetic (+)-(15S,16R)-1 proved to be significantly lower than the leaf extract containing naturally occurring (+)-(15S,16R)-16-hydroxy-16,22-dihydroapparicine.
Conclusion
We achieved the first total synthesis of (+)-(15S,16R)-16-hydroxy-16,22-dihydroapparicine (1) and the (−)-enantiomer and determined the absolute stereochemistry of naturally occurring 1. The synthesis involved a novel cascade reaction for efficient construction of the 1-azabicyclo[4.2.2]decane, including a pseudo-aminal moiety, via a Staudinger reaction, N-allylation, aza-Wittig reaction, and Mannich reaction. In addition, we developed a new method using diastereoselective 1,2-addition of methylketone, using N-TBSOM protecting the indole nucleophile and intramolecular chirality transferring Michael reaction with neighboring group participation. In particular, intramolecular chirality transferring Michael reaction proved to be a useful method for synthesis of the chiral tri-substituted butyrolactone. We established an effective enantioselective synthetic route for the production of pseudo-aminal alkaloids.
Synthetic (+)-(15S,16R)-1 exhibited moderate/weak antimalarial activity against chloroquine-resistant Plasmodium falciparum parasites and there is a possibility that the structurally unique compounds may be useful for the development of novel antimalarial drug candidates.
References
Ōmura S (1992) The search for bioactive compounds from microorganisms. Brock/Springer series in contemporary biosciences. Springer-Verlag, New York
Ōmura S (2015) Splendid gifts from microorganisms, 5th edn. The Kitasato Institute, Tokyo
Ishiyama A, Iwatsuki M, Namatame M, Nishihara-Tsukashima A, Sunazuka T, Takahashi Y, Ōmura S, Otoguro K (2011) Borrelidin, a potent antimalarial: stage-specific inhibition profile of synchronized cultures of Plasmodium falciparum. J Antibiot (Tokyo) 64:381–384
World Health Organization (WHO) (2014) World malaria report 2014. Available online at: http://www.who.int/malaria/publications/world_malaria_report_2014/report/en/. Accessed 1 Apr 2016
Ridley RG (2002) Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 415:686–693
Greenwood BM, Fidock DA, Kyle DE, Kappe SHI, Alonso PL, Collins FH, Duffy PE (2008) Malaria: progress, perils, and prospects for eradication. J Clin Invest 118:1266–1276
Eastman RT, Fidock DA (2009) Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol 7:864–874
Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NPJ, Lindegardh N, Socheat D, White NJ (2009) Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467
Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM; Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium (2008) Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359:2619–2620
White NJ (2008) Qinghaosu (artemisinin): the price of success. Science 320:330–334
Ward SA, Sevene EJ, Hastings IM, Nosten F, McGready R (2007) Antimalarial drugs and pregnancy: safety, pharmacokinetics, and pharmacovigilance. Lancet Infect Dis 7:136–144
Olliaro P, Wells TN (2009) The global portfolio of new antimalarial medicines under development. Clin Pharmacol Ther 85:584–595
Wells TN, Alonso PL, Gutteridge WE (2009) New medicines to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov 8:879–891
Rottmann M, McNamara C, Yeung BKS, Lee MCS, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, González-Páez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT (2010) Spiroindolones, a potent compound class for the treatment of Malaria. Science 329:1175–1180
Winzeler EA (2008) Malaria research in the post-genomic era. Nature 455:751–756
Ioset JR (2008) Natural products for neglected diseases: a review. Curr Org Chem 12:643–666
Perera P, van Beek TA, Verpoorte R (1984) 16(S)-Hydroxy-16,22-dihydroapparicine, a new alkaloid from the leaves of Tabernaemontana dichotoma. J Nat Prod 47:835–838
Noguchi Y, Hirose T, Furuya Y, Ishiyama A, Otoguro K, Ōmura S, Sunazuka T (2012) The first total synthesis and reassignment of the relative stereochemistry of 16-hydroxy-16,22-dihydroapparicine. Tetrahedron Lett 53:1802–1807
Hirose T, Noguchi Y, Furuya Y, Ishiyama A, Iwatsuki M, Otoguro K, Ōmura S, Sunazuka T (2013) Structure determination and total synthesis of (+)-16-hydroxy-16,22-dihydroapparicine. Chem Eur J 19:10741–10750
Gilbert B, Duarte AP, Nakagawa Y, Joule JA, Flores SE, Aguayo Brissolese J, Campello J, Carrazzoni EP, Owellen RJ, Blossey EC, Brown KS Jr, Djerassi C (1965) Alkaloid studies. L. The alkaloids of twelve Aspidosperma species. Tetrahedron 21:1141–1166
Joule JA, Monteiro H, Durham LJ, Gilbert B, Djerassi C (1965) Alkaloid studies. 48. The structure of apparicine, a novel Aspidosperma alkaloid. J Chem Soc Perkin 1 4773–4780
Akhter L, Brown RT, Moorcroft D (1978) 10-Hydroxy- and 10-methoxyapparicine: two new alkaloids from Ochrosia oppositifolia. Tetrahedron Lett 19:4137–4140
Atta-ur-Rahman, Muzaffar A (1985) The isolation and structure of ervaticine, a new indole alkaloid from Ervatamia coronaria. Heterocycles 23:2975–2978
Kam TS, Pang HS, Choo YM, Komiyama K (2004) Biologically active ibogan and vallesamine derivatives from Tabernaemontana divaricata. Chem Biodivers 1:646–656
Michel S, Tillequin F, Koch M (1986) Brafouédine et Isobrafouédine: nouveaux alcaloïdes indoliques mineurs de Strychnos dinklagei. J Nat Prod 49:452–455
Pawelka KH, Stöckigt J, Danieli B (1986) Epchrosine—a new indole alkaloid isolated from plant cell cultures of Ochrosia elliptica Labill. Plant Cell Rep 5:147–149
Walser A, Djerassi C (1964) Alkaloid-studien XLIX die strukturen von Vallesamin und O-acetyl-vallesamin. Helv Chim Acta 47:2072–2086
Atta-ur-Rahman, Alvi KA, Abbas SA, Voelter W (1987) Isolation of 19,20-Z-vallesamine and 19,20-E-vallesamine from Alstonia scholaris. Heterocycles 26:413–419
Zeches M, Ravao T, Richard B, Massiot G, Le Men-Olivier L, Verpoorte R (1987) Some new vallesamine-type alkaloids. J Nat Prod 50:714–720
Yamauchi T, Abe F, Padolina WG, Dayrit FM (1990) Alkaloids from leaves and bark of Alstonia scholaris in the Philippines. Phytochemistry 29:3321–3325
Ku WF, Tan SJ, Low YY, Komiyama K, Kam TS (2011) Angustilobine and andranginine type indole alkaloids and an uleine–secovallesamine bisindole alkaloid from Alstonia angustiloba. Phytochemistry 72:2212–2218
Macabeo APG, Krohn K, Gehle D, Read RW, Brophy JJ, Cordell GA, Franzblau SG, Aguinaldo AM (2005) Indole alkaloids from the leaves of Philippine Alstonia scholaris. Phytochemistry 66:1158–1162
van Beek TA, Deelder AM, Verpoorte R, Svendsen AB (1984) Antimicrobial, antiamoebic and antiviral screening of some Tabernaemontana species. Planta Med 50:180–185
van Beek TA, Verpoorte R, Svendsen AB, Fokkens R (1985) Antimicrobially active alkaloids from Tabernaemontana chippii. J Nat Prod 48:400–423
van der Heijden R, Brouwer RL, Verpoorte R, van Beek TA, Harkes PAA, Svendsen AB (1986) Indole alkaloids from Tabernaemontana elegans. Planta Med 52:144–147
Ingkaninan K, Ijzerman AP, Taesotikul T, Verpoorte R (1999) Isolation of opioid-active compounds from Tabernaemontana pachysiphon leaves. J Pharm Pharmacol 51:1441–1446
Tarselli MA, Raehal KM, Brasher AK, Streicher JM, Groer CE, Cameron MD, Bohn LM, Micalizio GC (2011) Synthesis of conolidine, a potent non-opioid analgesic for tonic and persistent pain. Nat Chem 3:449–453
Lim KH, Low YY, Kam TS (2006) Biomimetic oxidative transformations of pericine: partial synthesis of apparicine and valparicine, a new pentacyclic indole alkaloid from Kopsia. Tetrahedron Lett 47:5037–5039
Bennasar ML, Zulaica E, Solé D, Alonso S (2009) The first total synthesis of (±)-apparicine. Chem Commun (23):3372–3374
Bennasar ML, Zulaica E, Solé D, Roca T, García-Díaz D, Alonso S (2009) Total synthesis of the bridged indole alkaloid apparicine. J Org Chem 74:8359–8368
Takanashi N, Suzuki K, Kitajima M, Takayama H (2016) Total synthesis of conolidine and apparicine. Tetrahedron Lett 57:375–378
Scopes DIC, Allen MS, Hignett GJ, Wilson NDV, Harris M, Joule JA (1977) A synthetic approach to the indole alkaloid apparicine. Synthesis of the ring skeleton. J Chem Soc Perkin Trans 1 (21):2376–2385
Kettle JG, Roberts D, Joule JA (2010) Synthesis of 1,2,3,4,5,7-hexahydro-6H-azocino[4,3-b]indol-6-ones as intermediates for the synthesis of apparicine. Heterocycles 82:349–370
Chauhan PS, Weinreb SM (2014) Convergent approach to the tetracyclic core of the apparicine class of indole alkaloids via a key intermolecular nitrosoalkene conjugate addition. J Org Chem 79:6389–6393
Martin CL, Nakamura S, Otte R, Overman LE (2011) Total synthesis of (+)-condylocarpine, (+)-isocondylocarpine, and (+)-tubotaiwine. Org Lett 13:138–141
Hurt CR, Lin R, Rapoport H (1999) Enantiospecific synthesis of (R)-4-amino-5-oxo-1,3,4,5-tetrahydrobenz[cd]indole, an advanced intermediate containing the tricyclic core of the ergots. J Org Chem 64:225–233
Hart DJ, Magomedov N (1999) Spiroquinazoline support studies: new cascade reactions based on the Morin rearrangement. J Org Chem 64:2990–2991
Wada Y, Nagasaki H, Tokuda M, Orito K (2007) Synthesis of N-protected staurosporinones. J Org Chem 72:2008–2014
Diker K, Döéde Maindreville M, Royer D, Provost FL, Lévy J (1999) The gramine route to the Diels–Alder adducts of indolo-2,3-quinodimethanes. Tetrahedron Lett 40:7463–7467
Somei M, Karasawa Y, Kaneko C (1981) Selective mono-alkylation of carbon nucleophiles with gramine. Heterocycles 16:941–949
Freed JD, Hart DJ, Magomedov NA (2001) Trapping of the putative cationic intermediate in the Morin rearrangement with carbon nucleophiles. J Org Chem 66:839–852
Low KH, Magomedov NA (2005) Phosphine-mediated coupling of gramines with aldehydes: a remarkably simple synthesis of 3-vinylindoles. Org Lett 7:2003–2005
Grubbs AW, Artman GD 3rd, Tsukamoto S, Williams RM (2007) A concise total synthesis of the notoamides C and D. Angew Chem Int Ed Engl 46:2257–2261
Artman GD 3rd, Grubbs AW, Williams RM (2007) Concise, asymmetric, stereocontrolled total synthesis of stephacidins A, B and notoamide B. J Am Chem Soc 129:6336–6342
Dubey R, Olenyuk B (2010) Direct organocatalytic coupling of carboxylated piperazine-2,5-diones with indoles through conjugate addition of carbon nucleophiles to indolenine intermediates. Tetrahedron Lett 51:609–612
de la Herrán G, Segura A, Csákÿ AG (2007) Benzylic substitution of gramines with boronic acids and rhodium or iridium catalysts. Org Lett 9:961–964
Csomós P, Fodor L, Sohár P, Bernáth G (2005) Synthesis of thiazino[6,5-b]indole derivatives, analogues of the phytoalexin cyclobrassinin. A new method for preparation of 3-aminomethylindole. Tetrahedron 61:9257–9262
Kennedy AR, Taday MH, Rainier JD (2001) The use of sulfur ylides in the synthesis of substituted indoles. Org Lett 3:2407–2409
Nishimura T, Yamada K, Takebe T, Yokoshima S, Fukuyama T (2008) (1-Nosyl-5-nitroindol-3-yl)methyl ester: a novel protective group for carboxylic acids. Org Lett 10:2601–2604
Shinohara H, Fukuda T, Iwao M (1999) A formal synthesis of optically active clavicipitic acids, unusual azepinoindole-type ergot alkaloids. Tetrahedron 55:10989–11000
Jones DT, Artman GD 3rd, Williams RM (2007) Coupling of activated esters to gramines in the presence of ethyl propiolate under mild conditions. Tetrahedron Lett 48:1291–1294
Staudinger H, Meyer J (1919) Über neue organische Phosphorverbindungen III. Phosphinmethylenderivate und Phosphinimine. Helv Chim Acta 2:635–646
Zimmer H, Singh G (1963) Synthesis of some triphenylphosphinalkylimines and mono- and dialkylaminotriphenylphosphonium halides. J Org Chem 28:483–486
Zimmer H, Jayawant M, Gutsch P (1970) Synthesis of secondary amines via triphenylphosphine imines. J Org Chem 35:2826–2828
Briggs EM, Brown GW, Jiricny J, Meidine MF (1980) Synthetic uses of iminophosphoranes. Monoalkylation of primary aromatic amines. Synthesis 1980:295–296
Frøyen P, Skramstad J (1998) Phosphorus in organic synthesis. The Tanigawa reaction revisited as a method for converting alcohols to tertiary amines. Tetrahedron Lett 39:6387–6390
Chérest M, Felkin H, Prudent N (1968) Torsional strain involving partial bonds. The stereochemistry of the lithium aluminium hydride reduction of some simple open-chain ketones. Tetrahedron Lett 9:2199–2204
Anh NT (1980) Regio- and stereo-selectivities in some nucleophilic reactions. Top Curr Chem 88:145–162
Anh NT, Eisenstein O (1977) Theoretical interpretation of 1–2 asymmetric induction-importance of anti-periplanarity. Nouv J Chim 1:61–70
Anh NT, Eisenstein O (1976) Induction asymetrique 1–2: comparaison ab initio des modeles de cram, de cornforth, de Karabatsos et de felkin. Tetrahedron Lett 17:155–158
Mengel A, Reiser O (1999) Around and beyond Cram’s rule. Chem Rev 99:1191–1224
Dambacher J, Anness R, Pollock P, Bergdahl M (2004) Highly diastereoselective conjugate additions of monoorganocopper reagents to chiral imides. Tetrahedron 60:2097–2110
Martinelli MJ (1990) Asymmetric Diels–Alder reaction with γ-functionalized α,β-unsaturated chiral N-acyloxazolidinones: synthesis of (+)-S-145. J Org Chem 55:5065–5073
Sundberg RJ, Russell HF (1973) Syntheses with N-protected 2-lithioindoles. J Org Chem 38:3324–3330
Mahboobi S, Uecker A, Sellmer A, Cénac C, Höcher H, Pongratz H, Eichhorn E, Hufsky H, Trümpler A, Sicker M, Heidel F, Fischer T, Stocking C, Elz S, Böhmer FD, Dove S (2006) Novel bis(1H-indol-2-yl)methanones as potent inhibitors of FLT3 and platelet-derived growth factor receptor tyrosine kinase. J Med Chem 49:3101–3115
Naka H, Akagi Y, Yamada K, Imahori T, Kasahara T, Kondo Y (2007) Fluorous synthesis of Yuehchukene by α-lithiation of perfluoroalkyl-tagged 1-(arylsulfonyl)indole with mesityllithium. Eur J Org Chem (28):4635–4637
Mahboobi S, Uecker A, Cénac C, Sellmer A, Eichhorn E, Elz S, Böhmer FD, Dove S (2007) Inhibition of FLT3 and PDGFR tyrosine kinase activity by bis(benzo[b]furan-2-yl)methanones. Bioorg Med Chem 15:2187–2197
Bourderioux A, Kassis P, Mérour JY, Routier S (2008) Synthesis of new fused and substituted benzo and pyrido carbazoles via C-2 (het)arylindoles. Tetrahedron 64:11012–11019
So CM, Yeung CC, Lau CP, Kwong FY (2008) A new family of tunable indolylphosphine ligands by one-pot assembly and their applications in Suzuki–Miyaura coupling of aryl chlorides. J Org Chem 73:7803–7806
Noguchi-Yachide T, Tetsuhashi M, Aoyama H, Hashimoto Y (2009) Enhancement of chemically-induced HL-60 cell differentiation by 3,3′-diindolylmethane derivatives. Chem Pharm Bull 57:536–540
Denton JR (2010) One-pot desulfonylative alkylation of N-sulfonyl azacycles using alkoxides generated by phase-transfer catalysis. Synthesis (5):775–782
Yasukochi T, Inaba C, Fukase K, Kusumoto S (1999) Nitropyridyl glycosides: new glycosyl donors for enzymatic transglycosylation. Tetrahedron Lett 40:6585–6589
Yasukochi T, Fukase K, Kusumoto S (1999) 3-Nitro-2-pyridyl glycoside as donor for chemical glycosylation and its application to chemoenzymatic synthesis of oligosaccharide. Tetrahedron Lett 40:6591–6593
Ballesteros P, Claramunt RM (1987) Study of the catalytic properties of tris(3,6-dioxaheptyl) amine (TDA-1) in heteroaromatic nucleophilic substitution of chloropyridines and their n-oxides. Tetrahedron 43:2557–2564
Nakano M, Kikuchi W, Matsuo JI, Mukaiyama T (2001) An efficient method for the p-methoxybenzylation of hydroxy group with 2-(4-methoxybenzyloxy)-3-nitropyridine. Chem Lett 30:424–425
Benneche T, Gundersen LL, Undheim K (1988) (tert-Butyldimethylsilyloxy)methyl chloride: synthesis and use as N-protecting group in pyrimidinones. Acta Chem Scand B 42:384–389
Gundersen LL, Benneche T, Undheim K (1989) Chloromethoxysilanes as protecting reagents for sterically hindered alcohols. Acta Chem Scand 43:706–709
Pitsch S, Weiss PA, Wu X, Ackermann D, Honegger T (1999) Fast and reliable automated synthesis of RNA and partially 2′-O-protected precursors (‘caged RNA’) based on two novel, orthogonal 2′-O-protecting groups. Helv Chim Acta 82:1753–1761
Pitsch S, Weiss PA, Jenny L, Stutz A, Wu X (2001) Reliable chemical synthesis of oligoribonucleotides (RNA) with 2′-O-[(triisopropylsilyl)oxy]methyl(2′-O-tom)-protected phosphoramidites. Helv Chim Acta 84:3773–3795
Zajac MA, Vedejs E (2004) A synthesis of the diazonamide heteroaromatic biaryl macrocycle/hemiaminal core. Org Lett 6:237–240
Attaluri S, Bonala RR, Yang IY, Lukin MA, Wen Y, Grollman AP, Moriya M, Iden CR, Johnson F (2010) DNA adducts of aristolochic acid II: total synthesis and site-specific mutagenesis studies in mammalian cells. Nucleic Acid Res 38:339–352
Hoffmann RW (1989) Allylic 1,3-strain as a controlling factor in stereoselective transformations. Chem Rev 89:1841–1860
Smith AB III, Sestelo JP, Dormer PG (1995) Total synthesis of (−)-furaquinocin C. J Am Chem Soc 117:10755–10756
Sestelo JP, Dormer PG (2000) A highly efficient synthetic route to (−)-furaquinocin C. Heterocycles 52:1315–1328
Greatrex BW, Kimber MC, Taylor DK, Fallon G, Tiekink ER (2002) 1,2-Dioxines as masked cis γ-hydroxy enones and their versatility in the synthesis of highly substituted γ-lactones. J Org Chem 67:5307–5314
Peña-López M, Martínez MM, Sarandeses LA, Pérez Sestelo J (2009) Total synthesis of (+)-neomarinone. Chem Eur J 15:910–916
Baldwin JE (1976) Rules for ring closure. J Chem Soc Chem Commun (18):734–736
Jung ME, Gervay J (1989) Solvent effects in intramolecular Diels–Alder reactions of 2-furfuryl methyl fumarates: evidence for a polar transition state. J Am Chem Soc 111:5469–5470
Yorimitsu H, Nakamura T, Shinokubo H, Oshima K, Omoto K, Fujimoto H (2000) Powerful solvent effect of water in radical reaction: triethylborane-induced atom-transfer radical cyclization in water. J Am Chem Soc 122:11041–11047
Li TT, Wu YL (1988) An approach to forskolin an efficient synthesis of a tricyclic lactone intermediate. Tetrahedron Lett 29:4039–4040
Somoza C, Darias J, Rúveda EA (1989) Intramolecular Michael–aldol condensation approach to the construction of advanced intermediates in the synthesis of forskolin. J Org Chem 54:1539–1543
Little RD, Masjedizadeh MR, Wallquist O, McLoughlin JI (1995) The intramolecular Michael reaction. Org React 47:315–552
Bacigaluppo JA, Colombo MI, Preite MD, Zinczuk J, Rúveda EA (1996) The Michael–aldol condensation approach to the construction of key intermediates in the synthesis of nimbolide and nagilactone A. Synth Commun 26:2737–2749
Uchida K, Ishigami K, Watanabe H, Kitahara T (2007) Synthesis of an insecticidal tetrahydroisocoumarin, (3R,4S,4aR)-4,8-dihydroxy-3-methyl-3,4,4a,5-tetrahydro-1H-2-benzopyran-1-one. Tetrahedron 63:1281–1287
Crow JR, Thomson RJ, Mander LN (2006) Synthesis and confirmation of structure for the gibberellin GA131 (18-hydroxy-GA4). Org Biomol Chem 4:2532–2544
Lainchbury MD, Medley MI, Taylor PM, Hirst P, Dohle W, Booker-Milburn KI (2008) A protecting group free synthesis of (±)-neostenine via the [5 + 2] photocycloaddition of maleimides. J Org Chem 73:6497–6505
Otoguro K, Kohana A, Manabe C, Ishiyama A, Ui H, Shiomi K, Yamada H, Ōmura S (2001) Potent antimalarial activities of polyether antibiotic, X-206. J Antibiot 54:658–663
Otoguro K, Ishiyama A, Ui H, Kobayashi M, Manabe C, Yan G, Takahashi Y, Tanaka H, Yamada H, Ōmura S (2002) In vitro and in vivo antimalarial activities of the monoglycoside polyether antibiotic, K-41 against drug resistant strains of Plasmodia. J Antibiot 55:832–834
Iwatsuki M, Takada S, Mori M, Ishiyama A, Namatame M, Nishihara-Tsukashima A, Nonaka K, Masuma R, Otoguro K, Shiomi K, Ōmura S (2011) In vitro and in vivo antimalarial activity of puberulic acid and its new analogs, viticolins A–C, produced by Penicillium sp. FKI-4410. J Antibiot 64:183–188
Acknowledgments
This work was supported by a grant for the 21st Century COE Program; a Grant-in-Aid for Young Scientists (22790017) to T.H. from the Ministry of Education, Culture, Sports, Science and Technology (MEXT); and a Kitasato University Research Grant for Young Researchers to T.H. We also thank Dr. Kenichiro Nagai and Ms. Noriko Sato (School of Pharmacy, Kitasato University) for their contributions. We are grateful to Dr. Toh-Seok Kam (University of Malaya) for providing an authentic natural sample of 16-hydroxy-16,22-dihydroapparicine.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Noguchi, Y., Hirose, T., Ishiyama, A. et al. Synthesis and stereochemical determination of an antiparasitic pseudo-aminal type monoterpene indole alkaloid. J Nat Med 70, 302–317 (2016). https://doi.org/10.1007/s11418-016-1012-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-016-1012-2