Abstract
Purpose
In Japan, the excavated soils produced from constructions projects, which contain relatively low levels of arsenic (As), are considered as a potential concern if they could release significant amount of As to the environment. The aim of this study was to investigate the As leaching from excavated alkaline soils and, in particular, the influence of drying methods, pH of extracting solution, and consecutive washing on As leaching from these soils.
Materials and methods
Four excavated alkaline soils obtained from different construction sites in Tokyo, Japan, were used in this study. The soils were pretreated by three drying methods (air-dried, 40 °C-dried, and freeze-dried). Sequential extraction procedure was applied to partition As into five operationally defined chemical fractions. Batch leaching tests (initial pH-controlled leaching test and consecutive washing test) were conducted to investigate the As release under different leaching conditions.
Results and discussion
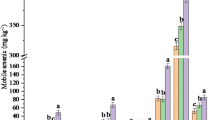
The As contents in the four soils were 9.22, 79.4, 6.75, and 11.7 mg kg−1, respectively, and As was primarily associated with the residual phase. Arsenic leaching was strongly dependent on the extracting solution pH values. Strongly acidic extracting solution (pH 2) led to circumneutral leachates and limited As mobility, whereas the strong alkaline-extracting solution (pH 12) greatly enhanced the As release from these soils. The consecutive washing test results revealed a long-term release of As from these excavated soils. The pollution potential indices (PPIs) were successfully used to evaluate the pollution threat of As leaching from excavated soils. In addition, different drying methods resulted in variations in the short- and long-term release of As from these excavated soils.
Conclusions
The results revealed that the soil pretreatment and the leaching conditions should be considered if we want to use batch tests for the contamination assessment of excavated urban soils from construction projects. Different drying methods and single extraction may lead to misestimation of the As pollution level. High extraction efficiency with strong alkaline-extracting solution (pH 12) reveals that it could potentially be used to wash As from excavated alkaline soils.




Similar content being viewed by others
References
Aiken GR, McKnight DM, Wershaw RL, MacCarthy P (eds) (1985) Humic substances in soil, sediment, and water: geochemistry, isolation and characterization. John Wiley & Sons, New York
Alam M, Tokunaga S (2006) Chemical extraction of arsenic from contaminated soil. J Environ Sci Health A Tox Hazard Subst Environ Eng 41:631–7643
Anawar HM, Akai J, Komaki K, Terao H, Yoshioka T, Ishizuka T, Safiullah S, Kato K (2003) Geochemical occurrence of arsenic in groundwater of Bangladesh: sources and mobilization processes. J Geochem Explor 77:109–131
Bauer M, Blodau C (2006) Mobilization of arsenic by dissolved organic matter from iron oxides, soils and sediments. Sci Total Environ 354:179–190
Bethke CM (1992) The Geochemist's workbench: a user’s guide to Rxn, Act2, tact, react and Gtplot. University of Illinois-Urbana, Illinois
Cappuyns V, Swennen R (2008a) The use of leaching tests to study the potential mobilization of heavy metals from soils and sediments: a comparison. Water Air Soil Pollut 191:95–111
Cappuyns V, Swennen R (2008b) The application of pH (stat) leaching tests to assess the pH-dependent release of trace metals from soils, sediments and waste materials. J Hazard Mater 158:185–195
Dung TTT, Golreihan A, Vassilieva E, Phung NK, Cappuyns V, Swennen R (2015) Insights into solid phase characteristics and release of heavy metals and arsenic from industrial sludge via combined chemical, mineralogical, and microanalysis. Environ Sci Pollut R 22:2205–2218
Fang K, Yuan DX, Zhang L, Feng LF, Chen YJ, Wang YZ (2015) Effect of environmental factors on the complexation of iron and humic acid. J Environ Sci-China 27:188–196
Grathwohl P, Susset B (2009) Comparison of percolation to batch and sequential leaching tests: theory and data. Waste Manag 29:2681–2688
Houben D, Evrard L, Sonnet P (2013) Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 92:1450–1457
Huang GX, Chen ZY, Sun JC, Liu F, Wang J, Zhang Y (2015) Effect of sample pretreatment on the fractionation of arsenic in anoxic soils. Environ Sci Pollut R 22:8367–8374
Imai N, Terashima S, Ohta M, Ujiie-Mikoshiba M, Okai T, Tachibana Y et al (2010) Geochemical map of sea and land of Japan. Geological Survey of Japan 207
Jang M, Hwang JS, Choi SI (2007) Sequantial soil washing techniques using hydrochloric acid and sodium hydroxide for remediating arsenic-contaminated soils in abandoned iron-ore mines. Chemosphere 66:8–17
Katsumi T (2015) Soil excavation and reclamation in civil engineering: environmental aspects. Soil Sci Plant Nutr 61:22–29
Kim EJ, Hwang BR, Baek K (2015) Effects of natural organic matter on the coprecipitation of arsenic with iron. Environ Geochem Hlth 37:1029–1039
Krüger O, Kalbe U, Berger W, Simon FG, Meza SL (2012) Leaching experiments on the release of heavy metals and PAH from soil and waste materials. J Hazard Mater 207−208:51–55
Lengke MF, Tempel RN (2005) Geochemical modeling of arsenic sulfide oxidation kinetics in a mining environment. Geochim Cosmochim Ac 69:341–356
Li JN, Kosugi T, Riya S, Hashimoto Y, Hou H, Terada A, Hosomi M (2016) Potential for leaching of arsenic from excavated rock after different drying treatments. Chemosphere 154:276–282
Luo W, Lu YL, Wang G, Shi YJ, Wang TY, Giesy JP (2008) Distribution and availability of arsenic in soils from the industrialized urban area of Beijing, China. Chemosphere 72:797–802
Mandal BK, Suzuki KT (2002) Arsenic around the world: a review. Talanta 58:201–235
Ministry of Land, Infrastructure, and Transport – MLIT (2014) Investigation results of construction by-products in 2012, http://www.mlit.go.jp/sogoseisaku/region/recycle/fukusanbutsu/jittaichousa/index01.htm (in Japanese)
Ministry of the Environment (2016) Soil Contamination Countermeasures Law. http://www.env.go.jp/water/dojo/law.html. Accessed 20 March 2016 (in Japanese)
Mohanty SK, Saiers JE, Ryan JN (2014) Colloid-facilitated mobilization of metals by freeze-thaw cycles. Environ Sci Technol 48:977–984
Molinari A, Guadagnini L, Marcaccio M, Straface S, Sanchez-Vila X, Guadagnini A (2013) Arsenic release from deep natural solid matrices under experimentally controlled redox conditions. Sci Total Environ 444:231–240
Notification No.46 of the Environment Agency (1991) Environmental quality for soil pollution. Ministry of the Environment, Japan
Oztas T, Fayetorbay F (2003) Effect of freezing and thawing processes on soil aggregate stability. Catena 52:1–8
Parkhurst DL, and Appelo CAJ (1999) User’s guide to PHREEQC (version 2) a computer program for speciation, batch-reaction, one-dimensional transport and inverse geochemical calculations. Water-Resources Investigations Report 99–4259. U.S. Department of the interior, U.S. Geological survey
Qi YB, Huang B, Darilek JL (2014) Effect of drying on heavy metal fraction distribution in rice paddy soil. PLoS One 9
Redman AD, Macalady DL, Ahmann D (2002) Natural organic matter affects arsenic speciation and sorption onto hematite. Environ Sci Technol 36:2889–2896
Sharma P, Rolle M, Kocar B, Fendorf S, Kappler A (2011) Influence of natural organic matter on As transport and retention. Environ Sci Technol 45:546–553
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Supriatin S, Terrones CA, Bussink W, Weng LP (2015) Drying effects on selenium and copper in 0.01 M calcium chloride soil extractions. Geoderma 255:104–114
Tabelin CB, Igarashi T (2009) Mechanisms of arsenic and lead release from hydrothermally altered rock. J Hazard Mater 169:980–990
Tabelin CB, Basri AHM, Igarashi T, Yoneda T (2012) Removal of arsenic, boron, and selenium from excavated rocks by consecutive washing. Water Air Soil Poll 223:4153–4167
Tabelin CB, Hashimoto A, Igarashi T, Yoneda T (2014a) Leaching of boron, arsenic and selenium from sedimentary rocks: I. Effects of contact time, mixing speed and liquid-to-solid ratio. Sci Total Environ 472:620–629
Tabelin CB, Hashimoto A, Igarashi T, Yoneda T (2014b) Leaching of boron, arsenic and selenium from sedimentary rocks: II. pH dependence, speciation and mechanisms of release. Sci Total Environ 473:244–253
Walker FP, Schreiber ME, Rimstidt JD (2006) Kinetics of arsenopyrite oxidative dissolution by oxygen. Geochim Cosmochim Ac 70:1668–1676
Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano DC (2001) Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta 436:309–323
Yu YM, Zhu YX, Gao ZM, Gammons CH, Li DX (2007) Rates of arsenopyrite oxidation by oxygen and Fe(III) at pH 1.8-12.6 and 15-45 degrees C. Environ Sci Technol 41:6460–6464
Acknowledgements
This research was supported by the Environment Research and Technology Development Fund of the Ministry of the Environment, Japan (5-1606). The XAFS spectroscopy experimentation was conducted using a Beamline BL14B2 at the SPring-8, Hyogo, Japan, supported by the Japan Synchrotron Radiation Research Institute (proposal numbers: 2015A1667 and 2015B1565). We also wish to thank the anonymous reviewers for their constructive comments that improved this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Dong-Mei Zhou
Electronic supplementary material
ESM 1
(DOCX 629 kb)
Rights and permissions
About this article
Cite this article
Li, J., Kosugi, T., Riya, S. et al. Use of batch leaching tests to quantify arsenic release from excavated urban soils with relatively low levels of arsenic. J Soils Sediments 17, 2136–2143 (2017). https://doi.org/10.1007/s11368-017-1669-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1669-5