Abstract
Purpose
Although a wide number of industrial processes routinely release radionuclides into the environment, the resulting potential impacts on human health have been largely overlooked in life cycle assessment (LCA). As part of the Life Cycle Initiative project on Global Guidance for Life Cycle Impact Assessment Indicators and Methods (GLAM), we aim to develop a consensus-based source-to-damage framework and factors for characterizing human health damage from ionizing radiation in LCA.
Methods
Our framework comprises four modules. The fate and exposure modules are based on UCrad, an earlier developed compartment-based environmental model for radionuclides. The focus of the present work is on the dose response and severity modules, which are based on most recent data from the International Committee on Radiological Protection and the Global Burden of Disease project series. The characterization factors are expressed in terms of DALY per kBq released.
Results and discussions
We obtain characterization factors for 115 radionuclides and 8 environmental compartments. To evaluate our approach, we compare both effect factors (combining dose response and severity) and characterization factors with those proposed in earlier studies. Our analysis demonstrates that differences are explicable by the different approaches used in the fate and exposure modelling. We also test the sensitivity of our factors to different approaches for filling data gaps, suggesting that our factors are robust. Finally, we apply our factors in an illustrative case study on rice production and consumption under various scenarios to identify dominant radionuclides and how these differ when other approaches are used.
Conclusions
Our framework is aligned with widely adopted methodologies for human health impact assessment, thus enabling robust comparisons, and covers nearly all radionuclides released by anthropogenic activities, including those that may arise from disposal of nuclear waste. Our factors are readily applicable for assessing radionuclide emissions in LCA. As next step we recommend (i) incorporating decay products into the fate model and (ii) integrating a model for indoor emissions of radon and indoor exposure to naturally occurring radionuclides (NORM).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
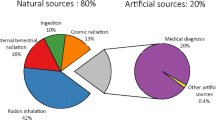
The term ionizing radiation refers to a form of high-energy radiation that is capable of detaching electrons from atoms or molecules, typically forming ions (hence the term “ionizing”) (UNEP 2016; US Nuclear Regulatory Commission 2021). This electron displacement may lead to changes in living cells and thus induce detrimental effects to humans and other organisms. The average radiation dose absorbed by humans is dominated by natural sources—i.e. the natural background radiation, which includes cosmic radiation, airborne radon, terrestrial sources like uranium and ingestion of other naturally occurring radioactive materials (NORM) (UNSCEAR 2008). However, human activities also contribute to ionizing radiation impacts by enhancing exposure to NORM, e.g. from the building sector and coal combustion, and by releasing man-made sources of ionizing radiations, primarily from the nuclear fuel cycle and nuclear medicine (UNSCEAR 2008).
Although a wide number of industrial processes routinely release radionuclides, the impacts of the resulting exposure to ionizing radiation have been often overlooked in life cycle assessment (LCA) studies, in part due to poor data availability and in part due to a lack of a methodology that enables accounting for most sources of ionizing radiation, particularly those arising from disposal of nuclear waste. A comprehensive review of methodologies for addressing radiological impacts in LCA, including how they are included in LCA impact assessment methods and their respective limitations, is provided by Paulillo et al. (2018). Heijungs (1994) developed the first approach to include ionizing radiation impacts in LCA: the critical volume approach; this lacked a fate model for dispersion of radionuclides in the environment and only accounted for the released amount compared to a maximum permissible concentration or quality standard in the receiving medium. Frischknecht et al. (2000) developed a methodology that included a fate and exposure model and also accounted for the potential damages to human health expressed in terms of disability-adjusted life years (DALY), a measure of loss of years of full health developed by the World Health Organization (WHO) as part of the Global Burden of Disease (GBD) project (Murray and Lopez 1996; WHO 2013). All current life cycle impact assessment (LCIA) methods that include ionizing radiation impacts use the methodology developed by Frischknecht et al. (2000) (Paulillo et al. 2018).
Other approaches have been proposed to address some of the limitations of Frischknecht et al.’s methodology, particularly focusing on the fate and exposure modelling and on expanding the coverage of ionizing radiation sources. Solberg-Johansen (1998) proposed an approach to include radiological impacts arising from nuclear waste, including those released from accidental (stochastic) events. Building on the work of Solberg-Johansen, Paulillo et al. (2020a, c) developed a compartment-based environmental model (UCrad) by adapting the scientific consensus model USEtox, which is widely used in LCA for characterizing human toxicity and ecotoxicity of chemicals (Rosenbaum et al. 2008; Westh et al. 2015). The UCrad model not only assesses radiological impacts arising from nuclear waste disposal, but also significantly extends the coverage of radionuclides to nearly all that are routinely released from human activities. Practical applications of UCrad can be found in Paulillo et al. (2021, 2020d) where it has been used to assess and compare disposal strategies for used nuclear fuels. Joyce et al. (2016) and Goronovski et al. (2018) developed an approach to account for radiological impacts from enhanced exposure to NORM, particularly focusing on indoor exposure, e.g. from building materials and radon. No recent work has focused on improving effect modelling, which is the conversion of radioactive exposure doses to human health damage.
To consolidate the state-of-the-art in addressing ionizing radiation impacts in LCA, the Life Cycle Initiative hosted by UN Environment brought together experts from the academic, private and public sectors to provide a consensus-based framework and factors for characterizing human health damages from radionuclide emissions, which have been identified as one of the priority impact categories in the frame of the Global Guidance for Life Cycle Impact Assessment Indicators and Methods (GLAM) (Jolliet et al. 2014, 2018; Frischknecht et al. 2016; Verones et al. 2017). The expert group aims at providing recommendations and the underlying methodological framework for addressing radionuclide emissions in LCA. In the present study, we propose such a framework by building upon earlier work to operationalize a full source-to-damage modelling approach for characterizing human health damage from ionizing radiation exposure within LCA. Key requirements of this framework are that it (i) covers most sources of ionizing radiation and (ii) is aligned with other widely adopted human health impact assessment methodologies used in LCA—e.g. the USEtox model for characterizing health damage from emissions of toxic chemicals (Fantke et al. 2018b, 2021) and models for characterizing health damage from indoor/outdoor fine particulate matter emissions (Fantke et al. 2015, 2017, 2019)—to enable robust comparisons. Hence, this study’s main goal is to propose a consistent set of human health damage characterization factors for a wide range of radioactive releases for use in LCA and other comparative assessments. To achieve this goal, we focus on three specific objectives: (i) to develop a source-to-damage framework for ionizing radiation, (ii) to combine a mass-balanced fate and exposure model with dose response and severity factors using the latest available data and (iii) to test the proposed framework in an illustrative case study on rice production and consumption and compare our results against results from previous studies.
2 Methodology
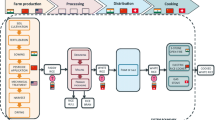
The radiological protection field has developed specific models to evaluate the actual impacts of radioactive emissions from a specific site, taking into account local environmental conditions (e.g. wind patterns) and the distribution and behaviour of the human population (e.g. IAEA 2001). Due to its global scope and whole system perspective, LCA relies on a broader set of parameterizations of, for example, locations and human populations. This implies that incorporating radiological impacts in LCA requires adapting more complex and site-specific models (Paulillo et al. 2018), to be consistent with the boundary conditions of LCA (Fantke et al. 2018a). Figure 1 schematically illustrates the proposed framework for characterizing human health damage from ionizing radiation, which is adapted from the work of Frischknecht et al. (2000) and aligned with consensus-based LCIA methodologies for toxic pollutants and fine particulate matter (Fantke et al. 2017, 2021). The framework focuses on releases of radionuclides; it can also be used to assess the impact of direct radiation exposures like those for medical purposes, but does not yet cover indoor radiation (e.g. from NORM or radon) which is still under development (see Sect. 4.2.1).
Proposed framework for characterizing human health impacts due to ionizing radiation. Blue steps build on the UCrad model, while orange steps are developed in the present work (adapted from Fantke et al. 2021)
The framework comprises four distinct modules (Fig. 1). Starting from a radioactive inventory mass that is emitted to the environment, the environmental fate module for the transport and dispersion of radionuclides estimates their mass at steady state in various environmental media. The exposure module estimates the radioactive dose absorbed by the human population via intake pathways such as inhalation and ingestion and external exposure like immersion in a radioactive plume. Estimating human health effects and related damage is covered by the last two modules: the dose response module estimates the population-level cumulative cancer incidence following an exposure, and the severity module translates this incidence into a measure of the overall burden of the disease expressed in terms of DALY. As shown in Fig. 1, each module is associated with a conversion factor; for example, the exposure factor (Sv/kBq) converts the steady-state mass of radionuclides in the environment into an average radioactive dose absorbed by humans. Following GLAM terminology, effect factors represent the product of dose response factors and severity factors. Note that for assessing the human health damage from direct radiation, only the last two modules are required, which are directly linked to the provided radiation dose.
We propose that the fate and exposure modules (in blue in Fig. 1) are based on the UCrad methodology developed by Paulillo et al. (2020a, c); in the present work, we focus in particular on the last two modules (in orange in Fig. 1) to update dose response and severity factors, as detailed in the following sections.
2.1 Fate and exposure
2.1.1 The UCrad methodology
The UCrad methodology was developed for assessing human impacts from ionizing radiation at midpoint level (i.e. Sv or risk).Footnote 1 We base the fate and exposure modules of our framework on UCradFootnote 2 for three main reasons. First, the methodology covers most (n=115) radionuclides released by anthropogenic activities, which is substantially more than other available methodologies (Sect. 1). Second, UCrad enables considering radiations arising from the disposal of nuclear waste, a potentially significant source (Paulillo et al. 2020d, 2021). Third, the methodology is fully aligned with USEtox so that robust comparisons can be made between human impacts from ionizing radiation and from toxic pollutants. Data used in UCrad fate and exposure modules, as well as the resulting midpoint characterization factors (CFs), are reported in Paulillo et al. (2020b).
UCrad is a multimedia environmental model at steady state (Mackay 2001). The fate module assumes continuous emissions and uses a system of coupled differential equations with first-order kinetics which are solved at steady state (i.e. balancing continuous emissions with continuous removals, which include decay and multimedia transfer processes) to estimate the amount of radionuclides (measured in Bq) in each compartment. The fate module is coupled with an exposure module to estimate the overall radiological dose absorbed by humans. This approach is similar to the fate modelling of multimedia models for toxic pollutants as done in USEtox.
The fate module uses a nested compartmental model comprised of two spatially nested scales—continental and global—and eight environmental compartments. In addition to the five compartments that are also considered in USEtox (i.e. air, fresh- and seawater, natural and agricultural soil), UCrad also includes freshwater and marine sediments and groundwater compartments. The sediment compartments contribute to external radiation to humans, while the groundwater compartment represents the receiving medium of radioactive releases arising from nuclear waste disposed in deep geological repositories. Groundwater is in fact recognized as the main pathway through which radionuclides from stored wastes can reach the biosphere (Radioactive Waste Management 2016). Note that it is not our goal to propose a model for estimating the potential radioactive releases specifically from a nuclear waste repository; instead, we aim to develop a methodology that can be used in combination with such models in a wider LCIA context (see Sect. 4.2). The sediment compartments are linked solely to the respective water compartment, and exchange between these compartments occurs by sedimentation/re-suspension and adsorption/desorption processes. The groundwater compartment is adapted from the GLOBOX model, which assumes that it is fed by water percolating from natural and agricultural soil and that it is linked to the ocean and freshwater through ground flows and to uncultivated and agricultural soils through irrigation (Wegener Sleeswijk and Heijungs 2010). Radioactive decay is included in UCrad as a removal process, with the fate of decay products currently ignored—this a current limitation in the fate module. Note however that the exposure to decay products after intake (i.e. the decay products that are generated after ingestion or inhalation) is accounted for by the dose conversion factors (ICRP 1981).
The exposure module considers two primary pathways: external and intake. Airborne and deposited radionuclides contribute to the external pathway, primarily via gamma radiation, while ingestion and inhalation constitute intake exposure pathways. The amount of radiation absorbed via external exposure is estimated considering the yearly average exposure time to a contaminated environment (IAEA 2001). The radiation dose absorbed via the intake pathway is based on consumption patterns of food and water and on the average human breathing rate, using factors from the USEtox model. The absorbed radiation dose is converted into an effective dose (which considers the biological effectiveness of the radiation and the biological tissue irradiated and is measured in Sieverts, Sv) using established conversion factors (Eckerman and Ryman 1993; IAEA 2011). The fate and exposure of carbon-14 (C14) and tritium (H3) require special consideration for two reasons (IAEA 2001). First, they are typically released in the form of CO2 and H2O (as THO or T2O), so UCrad fate module assumes these forms when estimating their environmental mass. Second, both radionuclides can be incorporated into a great variety of different chemical compounds, which makes exposure modelling complex; UCrad uses specific models (known as “specific activity models”) which are based on the assumption that the ratio between a radioactive nuclide and its widespread stable form is fixed at equilibrium (IAEA 2001).
2.2 Dose response and severity: approach and data sources
Our approach to develop the dose response and severity modules is based on the method originally proposed by Frischknecht et al. (2000). In our study, we update the underlying data and investigate approaches to fill gaps. In contrast to Frischknecht et al. (2000) and a later work by de Schryver et al. (2011), we do not consider discounting or age-weighting when assessing damage to human health; this follows the latest recommendations from GLAM (Frischknecht and Jolliet 2016). We also do not differentiate between short-term and long-term impacts because this requires considering dynamics in environmental fate and exposure processes. A differentiation between short- and long-term impacts, however, is possible at inventory level, where it is particularly relevant for radon emissions arising from uranium tailings (Frischknecht et al. 2000).
The dose response module estimates the incidence risk for different cancers in humans associated with an exposure to ionizing radiation. To this end, we use the latest risk coefficients developed by the International Committee on Radiological Protection (ICRP 2007), which reports the risks of developing a cancer case per tissue or organ irradiated. The severity factors estimate how severely the resulting cancer is damaging human health (i.e. how it affects human lifetime). For this, we use data from the Global Burden of Disease (GBD) 2019 study concerning incidence rate (cases across 10,000 persons) and resulting DALY (per case) for a wide number of neoplasms (Global Burden of Disease Collaborative Network 2021a); notably, DALY is a combination of two factors: Years of Life Lost (YLL) and Years Lived Disabled (YLD), both reported by the GBD project.
The development of severity factors required mapping cancer sites reported in the ICRP to the neoplasms classification used by the GBD. The mapping results are reported in Table S1 in the Supplementary Information (SI). The mapping exercise entailed addressing three problems, concerning how to deal with (i) missing neoplasms in the GBD database, (ii) multiple neoplasms being applicable to the same tissue or organ and (iii) heritable diseases.
Concerning the first aspect, we found no neoplasms in the GBD database that are applicable to bone tissue (known as “bone sarcoma”). For this tissue, we estimate DALY as the arithmetic mean of DALY of all neoplasms reported in the GBD weighted based on their prevalence. A prevalence-weighted approach ensures that the DALY is more affected by cancers that are currently more common, thus preventing the average from being excessively affected by unlikely but severe cancers like oesophageal cancer (Global Burden of Disease Collaborative Network 2021a). We adopted a similar approach for the case where multiple neoplasms are relevant for the same tissue (e.g. melanoma and non-melanoma skin cancers for skin irradiation), but in this case, the averaging is restricted to the relevant cancers. This approach also applies to what the ICRP refers to as “others solid” (ICRP 2007), which covers solid cancers in all other tissues and organs. In this case, the averaging covered all neoplasms that are not selected for other organs or tissues, excluding benign neoplasms. Since the averaging approach may affect the resulting severity factors, we conducted a sensitivity analysis using three alternative averaging approaches based on (i) an unweighted arithmetic mean, (ii) an incidence-weighted arithmetic mean and (iii) a median. Both prevalence and incidence figures are obtained from the GBD database (Global Burden of Disease Collaborative Network 2021a).
We used a different approach for heritable diseases, which are linked to irradiation of gonads and not included in the GBD. Using the lethality fraction reported by the ICRP, we assume that 80% of cases lead to a full lifetime lost, while 20% lead to a full lifetime lived with a disability. To estimate YLL, we use the representative life tables developed by the GBD (Global Burden of Disease Collaborative Network 2021b), while for estimating YLD, we consider a weight factor of 0.4 from Frischknecht et al. (2000).
2.3 Case study
We applied our proposed characterization framework for ionizing radiation in an illustrative case study on rice production and consumption, which was used for introducing other impact assessment methodologies as part of the GLAM project. Details about the case study are found elsewhere (Frischknecht et al. 2016). The functional unit (FU) corresponds to 1 kg of cooked white rice, which is assessed under three different scenarios. In the first scenario, rice is produced and processed in rural locations and distributed and cooked in urban locations of China (CN). In the second scenario, the entire process from rice production to cooking is done in rural locations of India (IN). In the third scenario, rice is produced and processed in rural locations of the USA and distributed and cooked in urban locations of Switzerland (US/CH). For ionizing radiation impacts on human health, the life cycle inventory of rice production and consumption for all scenarios included 24 unique radionuclides and 38 environmental flows to different compartments. The water compartment is unspecified for a large portion of emissions in the LCI data, which originates from the Ecoinvent database where this classification of water emissions is most common as a likely legacy of old datasets. Following an approach used in other impact categories, we assigned to unspecified water emissions the same factors that represent emissions to freshwater. The life cycle inventory does not include emissions from nuclear waste disposal (even though nuclear energy is considered as part of the electricity mix) because relevant data is scarce and not yet incorporated in LCA databases; this aspect is therefore not investigated in the present case study. Examples of LCA studies considering ionizing radiation impact from nuclear waste disposal are provided, e.g. by Paulillo et al. (2020d, 2021). The life cycle inventory (LCI) results for all considered radionuclides are provided in Table S6-S8 in the SI Excel file.
3 Results
3.1 Effect factors
Table 1 presents our dose response, severity and resulting effect factors (which are combination of dose response and severity). In the Supplementary Information (SI), we provide a detailed version of this table that includes all underlying YLL and YLD factors (Table S2), as well as the results of the sensitivity analysis on the averaging approach for individual (Table S3) and aggregated (Table S4) severity factors. We also report the midpoint (Sv/kBq) and the resulting endpoint (i.e. damage level; DALY/kBq) characterization factors (CFs) for each radionuclide in Table S9 and S10 in the SI Excel file.
Our approach yields an aggregated effect factor equal to 0.61 DALY/Sv; the YLL component of the DALY is dominant, contributing to more than 95% of the total value (SI, Table S2). Combining effect factors with fate and exposure factors, our endpoint CFs span over 15 orders of magnitude, from ~10−20 for groundwater releases of radon-222 to ~3 × 10−5 for soil releases of thorium-232 DALY per kBq released (Table S10).
As shown in Table 1, the highest effect factors include cancers linked to lung and heritable disease from gonads irradiation, which together account for over half of the overall effect factor. Lung cancers have a high dose response (the second highest after skin-related cancers) and severity (the third highest) factors. The effect factors for heritable diseases are primarily driven by their severity factor being the highest at ~78 DALY/cancer case, while their dose response factor is among the lowest. Skin-related cancers have the highest dose response factors (1000 cancer cases per 10,000 persons per Sv) but the lowest severity factor (0.53 DALY/cancer case), contributing to only 9%; the dose response factor is nearly ten times higher than the second- and third-highest dose response factors (corresponding to remainders and lung cancers). The lowest effect factors are found for bone, bladder, ovarian and thyroid cancers.
The sensitivity analysis shows that the aggregated severity factor is marginally affected by the averaging approach, although the individual severity factors for neoplasms corresponding to the same cancer sites may vary substantially when using different approaches. The prevalence-weighted approach yields the lowest effect factor, with difference ranging from 3% for the unweighted median and 12% for the unweighted arithmetic mean. For individual neoplasms, the largest differences between averaging approaches are found for bone sarcoma and other neoplasms where the prevalence-weighted average yields the lowest severity factor, between 33 and 335% for the former and between 21 and 149% for the latter. Note that similar incidence and prevalence-weighted factors indicate that the incidence and prevalence of the disease are comparable, for example for cancers linked to bone marrow (e.g. leukaemia, myeloma, lymphoma and hematopoietic neoplasms); they can also differ wildly, like in the case of “other” cancers. Using a similar logic, severity factors obtained via either or both approaches yield different results from the arithmetic mean and median approaches when the incidence and/or prevalence of the diseases are not uniformly distributed: for example, higher incidence/prevalence-weighted factors indicate the most detrimental neoplasms are also those that are more prevalent or have higher incidence, and vice versa.
3.2 Rice case study results
In Fig. 2, we show the results for each scenario of the rice case study. The charts report the radionuclide inventory (kBq/FU) on the x-axis, the endpoint CFs (DALY/kBq) on the y-axis and the resulting iso-impact score lines on the diagonal lines (DALY/FU), which are obtained by combining both inventory results and respective CFs. The radionuclides with the highest ionizing radiation impact scores are located on the top right of the charts, and those with the lowest on the bottom left. The impact score for each radionuclide is reported in Table S6-S8 in the SI Excel file.
The cumulative impact score ranges from 1.46 × 10−8 DALY/FU for the rural India scenario to 2.65 × 10−8 DALY/FU for the urban China scenario. Notably, our results are in the same order of magnitude of those obtained by Fantke et al. (2021) for toxic impacts (when direct exposure to chemicals in packaging is included) for the same case study. Our CFs cover all radioactive emissions included in the case study inventory with the exception of aggregated flows representing groups of radionuclides with similar properties like noble gases, and alpha or beta emitters.
For all scenarios, water emissions of uranium-238 (seawater), radium-226 (freshwater and seawater) and atmospheric emissions of polonium-210 dominate the ionizing radiation impacts, with scores in the order of 10−8 to 10−9 DALY/FU. Other notable emissions with similar impact scores include lead-210 (to air) for China and India scenario, thorium-232 (to air) for the China scenario and thorium-230 (to freshwater) for the USA-Switzerland scenario. Radioactive water emissions primarily originate from the production of chemicals like citric acid and phosphoric acid, which are used directly or indirectly throughout the life cycle of rice. On the other hand, radioactive air emissions originate from electricity production from hard coal and lignite.
Figure 2 also shows that the highest CFS are dominated by air emissions which feature 9 out of the 10 highest CF; the radionuclides with the highest CF include iodine-129, thorium-232, thorium-230 and polonium-210, which have values above 10−5 DALY/kBq released. The only exception is freshwater emissions of thorium-232, which has a CF of a similar magnitude. For all scenarios, the radioactive inventory is dominated by atmospheric emissions of radon-222 (~101 to ~102 kBq/FU) which are approximately two orders of magnitude higher than the second highest, i.e. water emissions of tritium (hydrogen-3). Radon-222 also features the lowest CF (~3 × 10−16 DALY/kBq), thus resulting in negligible impact scores. Radon-222 emissions arise from uranium tailings (a by-product of uranium mining) and are assumed to occur over thousands of years. If such long-term emissions are excluded, the life cycle inventory result for radon-222 drops by a factor of 40, making it comparable with tritium emissions, while the cumulative impact score remains essentially unaffected. On the other hand, the radionuclide with the lowest inventory corresponds to air emissions of plutonium-238 (~10−13 kBq/FU); similar to radon-222, this radionuclide has negligible contributions although it has a relatively high CF (~10−6 DALY/kBq).
4 Discussion
4.1 Comparison with Frischknecht et al.’s methodology
In Fig. 3, we compare our effect and CFs with those proposed by Frischknecht et al. (2000).
Comparison of effect factors (A) and characterization factors (B) proposed in the present work and by Frischknecht et al. (2000)
Figure 3A shows that the aggregated effect factor proposed by Frischknecht et al. (1.51 DALY/Sv) is approximately twice that of our proposed effect factor (0.61 DALY/Sv). The effect factors decreased for most neoplasms, with relative reductions ranging from 58% for “other solid” and up to 94% for bone marrow. The largest decrease in absolute terms applies to heritable diseases due to gonads irradiation, whose effect factors decreased by 0.41 DALY/Sv (73%), from 0.57 DALY/Sv in Frischknecht et al. to 0.16 DALY/Sv in the present work. In Frischknecht et al. the effect factor for heritable diseases accounted for over a third of the total; this is reduced to a fifth in our work. The main reason is that the dose response factor was significantly revised downwards by the ICRP in its latest publication, from 100 to 20 cases per 10,000 persons per Sv, which more than offsets the increase in lethality from 0.5 to 0.8. Another factor that contributed to reduction in the effect factor for heritable disease is the revised reference tables developed by the GBD which decreased from 88 to 81 years.
Although the severity factor for most neoplasms decreased, our estimates also yield increases for some neoplasms including those linked to the lung, liver and skin, which increased from 20% for lung cancer to 164% for liver cancer. The latter also represents the largest absolute increase by 0.04 DALY/Sv, from 0.02 DALY/Sv (~2% of total effect factor) to 0.06 DALY/Sv (~11%). In this case, the difference between our and Frischknecht et al. factors is driven by increases in both dose response factors and severity factors.
In the comparison of CFs in Fig. 3B, we quantify the average difference between ours and Frischknecht et al.’s CFs via the mean log deviation parameter (Paulillo et al. 2020c), which is defined as the average value of the base-10 logarithm of the ratio of the CFs of each radionuclide (equation is reported in the Supporting Information). On average, our CFs are approximately one order of magnitude higher than those of Frischknecht et al. (see Table S5). These results are in line with the comparison performed by Paulillo et al. (2020c) for the midpoint factors, thus indicating that the main difference between the two methodologies is linked to the fate and exposure module, rather than to the newly estimated effect factors. For completeness, we report in Fig. S1 (SI) the results for the rice case study obtained using the CFs developed by Frischknecht et al. (2000). For all scenarios, these are dominated by atmospheric emissions of carbon-14 followed by radon-222; these in fact represent the radionuclides with the largest difference between UCrad and Frischknecht et al.’s fate and exposure modules (Paulillo et al. 2020c).
4.2 Applicability, limitations and future work
Our CFs enable assessing the damage to human health from ionizing radiation in most conditions, using an approach that is aligned with other existing endpoint methods in LCIA. Our factors encompass 115 radionuclides, representing nearly all radionuclides that are commonly released by anthropogenic activities and, crucially, including those that could be released by disposal of radioactive waste. However, they do not cover flows representing groups of radionuclides (e.g. noble gases) which are common in LCA databases like Ecoinvent—most likely they are legacy flows part of old datasets. There are different approaches to derive CFs for radionuclides groups (e.g. weighted based on global emissions); these should be investigated as part of future works.
Our CFs cover emissions to eight generic environmental compartments. The factors are not yet geographically differentiated nor site-specific, implying that they cannot be used to e.g. estimate thresholds for individual or collective doses. For this, site-specific risk assessment studies are needed, for example, using the concept of the “critical group”; Paulillo et al. (2020c) developed an LCA-compatible methodology for such applications. Our factors do not yet cover indoor exposure to ionizing radiation (Goronovski et al. 2018), which include NORM used in building materials and radon (Sect. 4.2.1). To address such scenarios, we are developing an indoor compartment that is embedded in UCrad and in line with the approaches used in USEtox as part of our future work.
One of the key advantages of our CFs is their ability to assess human impacts associated with radioactive waste disposal in a deep geological repository, which is enabled by the addition of a groundwater compartment. Most risk assessment studies in fact recognize groundwater as the most likely pathway through which radionuclides from stored wastes can reach the biosphere (e.g. see Radioactive Waste Management (2016)). We note that to assess the potential radiological impact arising from radioactive waste, the LCA practitioner needs as part of inventory modelling to apply a “retardation” factor to the radioactive releases into the groundwater compartment; this factor accounts for the radioactive decay of radionuclides due to the time it takes for the leak to occur and the time it takes for the radionuclides to migrate from the geosphere to the biosphere. Paulillo et al. (2020c) developed midpoint characterization factors for different types of nuclear waste (e.g. High Level Waste) that embed retardation factors.
Our methodological approach is aligned with widely adopted approaches for LCIA proposed in the literature, and thus, our CFs are applicable within most endpoint LCIA methods. We articulate this in three steps. First, the framework we propose is adapted from an initial approach developed by Frischknecht et al. (2000), whose factors are already included in currently available LCIA methods. Second, the approach we use to estimate human health damages, which is based on data from the GBD project, is aligned with methods for assessing toxic chemical and fine particulate matter impacts, which are part of recent GLAM recommendations. Finally, the fate and exposure modules are adapted from those developed for toxic pollutants in USEtox; this is key in that it enables consistent comparison between toxic and radioactive pollutants.
Our endpoint CFs inherit the limitations of the underlying midpoint factors that have been discussed in detail in Paulillo et al. (2020a, c). The most notable limitation is arguably that the fate module does not account for the generation of decay products, but only for the actual radionuclides released, though we note that the impact of progeny nuclides is included in the exposure analysis (Sect. 2.1.2). This limitation is likely to result in an underestimation of the human impact and thus should be the primary focus for refining the proposed approach. Another limitation concerns the availability of physicochemical data for radionuclides, which are used for fate modelling purposes. Since data for most radionuclides are currently missing, Paulillo et al. (2020c) relied on proxy data or assumptions; future works should focus on improving the data coverage. Future work should focus on estimating the uncertainty of the fate and exposure factors.
Another potential limitation of our effect factors is that our estimates are based on the much debated and controversial linear no-threshold (LNT) approach. This assumes that human health impacts from exposure to ionizing radiation occur at low doses, have no minimum threshold and are proportional to the dose, even though humans have always been exposed to a background (mostly natural) radiation and data on radiological impacts are only available at high doses, e.g. from the Japanese populations exposed to nuclear explosions and their progeny. The LNT approach is widely adopted in the radiological protection field, including the International Commission on Radiological Protection (ICRP). However, this approach has also been criticized as being not fully supported by experimental evidence, including by The United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR 2015) and others (e.g. Allison 2015; Siegel et al. 2015). The use of different dose response models may substantially affect the damage estimates to human health from routine radioactive releases that are low in nature because they are regulated by strict limits and thus likely to be confounded with the natural background radiation. A source of uncertainty concerns the consistency between ICRP dose response factors and GBD severity factors. We addressed this via a sensitivity analysis, which demonstrated the overall robustness of our factors. The effect factor for heritable disease is also subject to significant uncertainty and is a major contributor to the overall uncertainty in the effect factors. Future work should hence focus on better estimating the potential severity factor of heritable diseases due to gonads irradiations.
4.2.1 Naturally occurring radioactive materials (NORM) and indoor radon exposures
An ongoing effort planned for implementation in future versions of UCrad will add the capacity to address indoor exposure to NORM incorporated into building materials and to radon. For NORM, we are building on the approach of Goronovski et al. (2018) who account for exposure of building inhabitants to external gamma radiation and radon inhalation from NORM, using the model of Meijer et al. (2005). We are revising this approach to make it consistent with the USEtox indoor-air model that was developed for fine particulate matter exposures indoors (Hodas et al. 2016; Fantke et al. 2019). We are also reviewing the building materials emissions factors from Meijer et al. (2005) to determine if there are needed updates.
For indoor radon exposures both from NORM and from subsurface soil, we are assessing how the existing Goronovski et al. (2018) approach can be updated to account for most recent recommendations in the radiological protection field (UNSCEAR 2006, 2016). Because radon occupational disease burdens from extraction industries have not yet been addressed in LCA, we are also reviewing guidance from UNSCEAR (2016, 2006) for developing characterization factors for these populations and life cycle activities.
5 Conclusions
We operationalized a source-to-damage framework for assessing human health damage from ionizing radiation that is consistent with widely adopted approaches for LCIA. Our framework improves upon that proposed by Frischknecht and colleagues by combining mass-balanced fate and exposure factors from UCrad with dose response and severity factors using the latest available data from ICRP and GBD. The resulting endpoint characterization factors (CFs) cover nearly all radionuclides that are released by anthropogenic emissions, including those that may arise from the disposal of radioactive waste.
Our CFs are on average one order of magnitude higher than those proposed by Frischknecht et al. This is mostly due to the fate and exposure modelling (UCrad), which has been discussed elsewhere. Even though our effect factors are about half of those proposed by Frischknecht et al. (2000), they only explain a small fraction of the difference between the endpoint CFs of both approaches. The results of the illustrative rice case study enable us to identify key radioactive emissions that, due to a combination of high inventory and high CFs, contribute the most to the aggregated impact scores. These are largely dominated by water emissions of radium-226 and uranium-238 and air emissions of polonium-210. When using the Frischknecht et al. (2000) factors, the results are dominated by atmospheric emission of carbon-14—the radionuclide that features the highest difference in fate and exposure modelling.
We identified several potential future improvements that could address some of the limitations of our framework and resulting factors. The most notable include (i) developing an “indoor” compartment model and (ii) improving the fate modelling to account for progeny radionuclide from radioactive decay. The sensitivity of our effect estimates to models different from the linear no-threshold (LNT) should also be investigated. Despite these limitations, our present approach constitutes a mature characterization framework for addressing ionizing radiation impacts on human health in LCA and other comparative assessment frameworks.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Notes
Although in the original article by Paulillo et al. (2020c), UCrad only refers to the fate module, here, we also include the exposure module under the same model name.
References
Allison W (2015) Nuclear is for life: a cultural revolution. Wade Allison Publishing
de Schryver AM, van Zelm R, Humbert S et al (2011) Value choices in life cycle impact assessment of stressors causing human health damage. J Ind Ecol 15:796–815. https://doi.org/10.1111/j.1530-9290.2011.00371.x
Eckerman KF, Ryman JC (1993) External exposure to radionuclides in air , water , and soil - Federal Guidance Report No.12
Fantke P, Aurisano N, Bare J et al (2018) Toward harmonizing ecotoxicity characterization in life cycle impact assessment. Environ Toxicol Chem 37:2955–2971. https://doi.org/10.1002/etc.4261
Fantke P, Aylward L, Bare J et al (2018) Advancements in life cycle human exposure and toxicity characterization. Environ Health Perspect 126:1–10. https://doi.org/10.1289/EHP3871
Fantke P, Chiu WA, Aylward L et al (2021) Exposure and toxicity characterization of chemical emissions and chemicals in products: global recommendations and implementation in USEtox. Int J Life Cycle Assess 26:899–915. https://doi.org/10.1007/s11367-021-01889-y
Fantke P, Jolliet O, Apte JS et al (2017) Characterizing aggregated exposure to primary particulate matter: recommended intake fractions for indoor and outdoor sources. Environ Sci Technol 51:9089–9100. https://doi.org/10.1021/acs.est.7b02589
Fantke P, Jolliet O, Evans JS et al (2015) Health effects of fine particulate matter in life cycle impact assessment: findings from the Basel Guidance Workshop. Int J Life Cycle Assess 20:276–288. https://doi.org/10.1007/s11367-014-0822-2
Fantke P, McKone TE, Tainio M et al (2019) Global effect factors for exposure to fine particulate matter. Environ Sci Technol 53:6855–6868. https://doi.org/10.1021/acs.est.9b01800
Frischknecht R, Braunschweig A, Hofstetter P, Suter P (2000) Human health damages due to ionising radiation in life cycle impact assessment. Environ Impact Assess Rev 20:159–189
Frischknecht R, Fantke P, Tschümperlin L et al (2016) Global guidance on environmental life cycle impact assessment indicators: progress and case study. Int J Life Cycle Assess 21:429–442. https://doi.org/10.1007/s11367-015-1025-1
Frischknecht R, Jolliet O (2016) Global guidance for life cycle impact assessment indicators volume 1
Global Burden of Disease Collaborative Network (2021a) Risk factors-attributable cancer burden estimates 2010–2019. Seattle, United States of America.
Global Burden of Disease Collaborative Network (2021b) Global Burden of Disease Study 2019 (GBD 2019) Reference Life Table. Seattle, United States of America.
Goronovski A, Joyce PJ, Björklund A et al (2018) Impact assessment of enhanced exposure from Naturally Occurring Radioactive Materials (NORM) within LCA. J Clean Prod 172:2824–2839. https://doi.org/10.1016/j.jclepro.2017.11.131
Heijungs R (1994) Life cycle impact assessment A brief survey with some ideas on radiation. Paper presented at the technical committee meeting on development and use of environmnetal impact indicators for comparative risk assessment of different energy sources.
Hodas N, Loh M, Shin HM et al (2016) Indoor inhalation intake fractions of fine particulate matter: review of influencing factors. Indoor Air 26:836–856. https://doi.org/10.1111/ina.12268
IAEA (2001) Generic models for use in assessing the impact of discharges of radioactive substances to the environment. Safety Reports Series No. 19. IAEA, Vienna
IAEA (2011) Radiation protection and safety of radiation sources: international basic safety standards. IAEA Safety Sandards Series No. GSR Part 3.
ICRP (1981) Annals of the ICRP. ICRP Publication 69. Age-dependent doses to members of the public from intake of radionuclides: Part 3 Ingestion Dose Coefficients.
ICRP (2007) The 2007 recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 37:. https://doi.org/10.1016/j.icrp.2004.12.002
Jolliet O, Antón A, Boulay AM et al (2018) Global guidance on environmental life cycle impact assessment indicators: impacts of climate change, fine particulate matter formation, water consumption and land use. Int J Life Cycle Assess 23:2189–2207. https://doi.org/10.1007/s11367-018-1443-y
Jolliet O, Frischknecht R, Bare J et al (2014) Global guidance on environmental life cycle impact assessment indicators: findings of the scoping phase. Int J Life Cycle Assess 19:962–967. https://doi.org/10.1007/s11367-014-0703-8
Joyce PJ, Goronovski A, Tkaczyk AH, Björklund A (2016) A framework for including enhanced exposure to naturally occurring radioactive materials (NORM) in LCA. Int J Life Cycle Assess 1–18. https://doi.org/10.1007/s11367-016-1218-2
Mackay D (2001) Multimedia environmental models: the fugacity approach, 2nd ed. Lewis Publisher
Meijer A, Huijbregts MAJ, Reijnders L (2005) Human health damages due to indoor sources of organic compounds and radioactivity in life cycle impact assessment of dwellings - Part 1: Characterisation factors. Int J Life Cycle Assess 10:309–316. https://doi.org/10.1065/lca2004.12.194.1
Murray CJL, Lopez AD (1996) The global burden of disease. WHO, World Bank and Harvard School of Public Health, Boston
Paulillo A, Clift R, Dodds J et al (2018) Radiological impact assessment approaches for life cycle assessment studies: a review and possible ways forward. Environ Rev 26:239–254. https://doi.org/10.1139/er-2018-0004
Paulillo A, Clift R, Dodds J et al (2020a) Radiological impacts in life cycle assessment – Part II: Comparison of methodologies. Sci Total Environ 708:134712. https://doi.org/10.1016/j.scitotenv.2019.134712
Paulillo A, Clift R, Dodds J et al (2020b) Data supporting UCrad and CGM, two novel methodologies for radiological impacts in Life Cycle Assessment. Data Br 28:104857. https://doi.org/10.1016/j.dib.2019.104857
Paulillo A, Clift R, Dodds JM et al (2020c) Radiological impacts in life cycle assessment. Part I: General framework and two practical methodologies. Sci Total Environ 708:135179. https://doi.org/10.1016/j.scitotenv.2019.135179
Paulillo A, Dodds JM, Milliken A et al (2020d) The environmental impacts of reprocessing Used Nuclear Fuels: a UK case study. Sustain Mater Technol 25:. https://doi.org/10.1016/j.susmat.2020.e00186
Paulillo A, Dodds JM, Palethorpe SJ, Lettieri P (2021) Reprocessing vs direct disposal of used nuclear fuels: the environmental impacts of future scenarios for the UK. Sustain Mater Technol 28:. https://doi.org/10.1016/j.susmat.2021.e00278
Radioactive Waste Management (2016) Geological disposal. Generic Post-closure Safety Assessment Geological Disposal. NDA Report no. DSSC/321/01 Geological
Rosenbaum RK, Bachmann TM, Gold LS et al (2008) USEtox - The UNEP-SETAC toxicity model: recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int J Life Cycle Assess 13:532–546. https://doi.org/10.1007/s11367-008-0038-4
Siegel JA, Pennington CW, Sacks B, Welsh JS (2015) The Birth of the Illegitimate Linear No-Threshold Model. Am J Clin Oncol 41:173–177. https://doi.org/10.1097/COC.0000000000000244
Solberg-Johansen B (1998) Environmental life cycle assessment of the nuclear fuel cycle. PhD thesis. University of Surrey
UNEP (2016) Radiation effects and sources
UNSCEAR (2006) Effects of ionizing radiation. Volume II: Scientific Annexes C, D and E. New York
UNSCEAR (2008) Source and effects of ionizing radiation. Annex B: Exposures of the public and workers from various sources of radiation. United Nations
UNSCEAR (2015) Sources, effects and risks of ionizing radiation. United Nations Scientific Committee on the Effects of Atomic Radiation 2012 Report to the General Assemby with Scientific Annexes. Annex A
UNSCEAR (2016) Sources, effects, and risks of ionizing radiation. United Nations Scientific Committee on the Effects of Ionizing Radiation 2016 report to the General Assembly, Scientific Annexes A, B, C, D. New York
US Nuclear Regulatory Commission (2021) Ionizing radiation. https://www.nrc.gov/reading-rm/basic-ref/glossary/ionizing-radiation.html
Verones F, Bare J, Bulle C et al (2017) LCIA framework and cross-cutting issues guidance within the UNEP-SETAC Life Cycle Initiative. J Clean Prod 161:957–967. https://doi.org/10.1016/j.jclepro.2017.05.206
Wegener Sleeswijk A, Heijungs R (2010) GLOBOX: a spatially differentiated global fate, intake and effect model for toxicity assessment in LCA. Sci Total Environ 408:2817–2832. https://doi.org/10.1016/j.scitotenv.2010.02.044
Westh TB, Hauschild MZ, Birkved M et al (2015) The USEtox story: a survey of model developer visions and user requirements. Int J Life Cycle Assess 20:299–310. https://doi.org/10.1007/s11367-014-0829-8
WHO (2013) WHO methods and data sources for global burden of disease estimates 2000-2011 86
Acknowledgements
The authors would like to thank Dr. Mikolaj Owsianiak for his input to the rice case study and all members of the UNEP GLAM Ionizing Radiation taskforce for their input into the consensus-building process.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Ralph K. Rosenbaum.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paulillo, A., McKone, T.E. & Fantke, P. Characterizing human health damage from ionizing radiation in life cycle assessment. Int J Life Cycle Assess 28, 1723–1734 (2023). https://doi.org/10.1007/s11367-023-02226-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-023-02226-1