Abstract
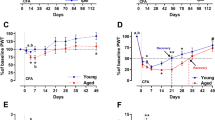
The aged population has a higher probability of developing chronic pain from acute insults because of age-associated low-grade inflammation. Several emerging studies have shown a crucial role of cap-dependent translation in the development of chronic pain in young adult animals; however, its role in the aged has never been reported. Acute and chronic inflammatory responses, including pain, are altered over age, and understanding how cap-dependent translation can represent an important and druggable pathway is imperative for understanding its therapeutic potential. Here we have tested how an inflammatory stimulus, complete Freund’s adjuvant (CFA), affects spontaneous and evoked pain, as well as inflammation in young versus aged mice that lack functional cap-dependent translation machinery (eukaryotic translation initiation factor 4E (eIF4E)) compared with age-matched wild-type (WT) mice. Interestingly, we found that CFA-induced acute pain and inflammation are modulated by eIF4E phosphorylation in aged but not young animals. Aged transgenic animals showed attenuated paw temperature and inflammation, as well as a mitigation in the onset and quicker resolution in mechanical and thermal hypersensitivity. We found that levels of interleukin (IL)-1β and tumor necrosis factor (TNF)-α are elevated in dorsal root ganglia in aged WT and eIF4E transgenic groups, despite faster resolution of acute inflammation and pain in the aged eIF4E transgenic animals. We propose that these cytokines are important in mediating the observed behavioral responses in the young and represent an alternate pathway in the development of age-associated inflammation and behavioral consequences. These findings demonstrate that eIF4E phosphorylation can be a key target for treating inflammatory pain in the aged.







Similar content being viewed by others
Data availability
All data gathered and presented here as well as raw data will be made available for review upon request.
References
Aguilar-Valles, et al. Translational control of depression-like behavior via phosphorylation of eukaryotic translation initiation factor 4E. Nat Commun. 2018;9(1):1–14.
Amorim IS, Lach G, Gkogkas CG. The role of the eukaryotic translation initiation factor 4E (eIF4E) in neuropsychiatric disorders. Front Genet. 2018a;9:561. Published 2018 Nov 23. https://doi.org/10.3389/fgene.2018.00561.
Amorim IS, Kedia S, Kouloulia S, Simbriger K, Gantois I, Jafarnejad SM, et al. Loss of eIF4E phosphorylation engenders depression-like behaviors via selective mRNA translation. J Neurosci. 2018b;38(8):2118–33.
Burton MD, Johnson RW. Interleukin-6 trans-signaling in the senescent mouse brain is involved in infection-related deficits in contextual fear conditioning. Brain Behav Immun. 2012;26(5):732–8.
Buxade M, Parra-Palau JL, Proud CG. The Mnks: MAP kinase-interacting kinases (MAP kinase signal-integrating kinases). Front Biosci. 2008;13:5359–73.
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1).
Chung HY, Kim DH, Lee EK, Chung KW, Chung S, Lee B, et al. Redefining chronic inflammation in aging and age-related diseases: proposal of the senoinflammation concept. Aging Dis. 2019;10(2):367–82.
Cruz-Almeida Y, Aguirre M, Sorenson HL, Tighe P, Wallet SM, Riley JL III. Age differences in cytokine expression under conditions of health using experimental pain models. Exp Gerontol. 2015;72:150–6.
Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36)36):1001–1006. Published 2018 Sep 14. https://doi.org/10.15585/mmwr.mm6736a2.
Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84(4):932–9.
Ellis A, Bennett DL. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 2013;111(1)1):26–37. https://doi.org/10.1093/bja/aet128.
Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol. 2010;226(1–2):181–4.
Furic L, Rong L, Larsson O, Koumakpayi IH, Yoshida K, Brueschke A, et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci U S A. 2010;107(32):14134–9.
Garner KM, Amin R, Johnson RW, Scarlett EJ, Burton MD. Microglia priming by interleukin-6 signaling is enhanced in aged mice. J Neuroimmunol. 2018;324:90–9.
Gonskikh Y, Polacek N. Alterations of the translation apparatus during aging and stress response. Mech Ageing Dev. 2017;168:30–6.
Gubbels Bupp MR, Potluri T, Fink AL, Klein SL. The confluence of sex hormones and aging on immunity. Front Immunol. 2018;99:1269. Published 2018 Jun 4. https://doi.org/10.3389/fimmu.2018.01269.
Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. https://doi.org/10.1016/0304-3959(88)90026-7.
Herdy B, Jaramillo M, Svitkin YV, Rosenfeld AB, Kobayashi M, Walsh D, et al. Translational control of the activation of transcription factor NF-κB and production of type I interferon by phosphorylation of the translation factor eIF4E. Nat Immunol. 2012;13(6):543–50.
Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493(7432):338–45.
Joshi S, Platanias LC. Mnk kinase pathway: cellular functions and biological outcomes. World J Biol Chem. 2014;5(3):321–33.
Khoutorsky A, Price TJ. Translational control mechanisms in persistent pain. Trends Neurosci. 2018;41(2):100–14.
Kubben N, Misteli T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nature reviews. Mol Cell Biol. 2017;18(10):595–609. https://doi.org/10.1038/nrm.2017.68.
Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7(6):447–449. https://doi.org/10.1038/nmeth.1455.
Mangold CA, Wronowski B, du M, Masser DR, Hadad N, Bixler GV, Brucklacher RM, Ford MM, Sonntag WE, Freeman WM. Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J Neuroinflammation. 2017;14(1):141. https://doi.org/10.1186/s12974-017-0920-8.
Megat S, Ray PR, Moy JK, Lou TF, Barragán-Iglesias P, Li Y, et al. Nociceptor translational profiling reveals the Ragulator-Rag GTPase complex as a critical generator of neuropathic pain. J Neurosci. 2019;39(3):393–411.
Merrick WC, Pavitt GD. Protein synthesis initiation in eukaryotic cells. Cold Spring Harb Perspect Biol. 2018;10(12)12):a033092. https://doi.org/10.1101/cshperspect.a033092.
Moy JK, Khoutorsky A, Asiedu MN, Black BJ, Kuhn JL, Barragán-Iglesias P, et al. The MNK–eIF4E signaling axis contributes to injury-induced nociceptive plasticity and the development of chronic pain. J Neurosci. 2017;37(31):7481–99.
Moy JK, Kuhn JL, Szabo-Pardi TA, Pradhan G, Price TJ. eIF4E phosphorylation regulates ongoing pain, independently of inflammation, and hyperalgesic priming in the mouse CFA model. Neurobiol Pain. 2018a;4:45–50.
Moy JK, Khoutorsky A, Asiedu MN, Dussor G, Price TJ. eIF4E phosphorylation influences Bdnf mRNA translation in mouse dorsal root ganglion neurons. Front Cell Neurosci. 2018b;12:29.
Norden DM, Godbout JP. Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013;39(1):19–34.
Perkins AE, Piazza MK, Deak T. Stereological analysis of microglia in aged male and female Fischer 344 rats in socially-relevant brain regions. Neuroscience. 2018;377:40–52.
Pinho-Ribeiro F, Verri W, Chiu I. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017;38(1):5–19. https://doi.org/10.1016/j.it.2016.10.001.
Price TJ, Gold MS. From mechanism to cure: renewing the goal to eliminate the disease of pain. Pain Med. 2018;19(8):1525–49.
Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. https://doi.org/10.3389/fimmu.2018.00586.
Roberts, A.W., et al. The population 65 years and older in the United States. 2018.
Rostock C, Schrenk-Siemens K, Pohle J, Siemens J. Human vs. mouse nociceptors – similarities and differences. Neuroscience. 2018;387:13–27.
Rowlett R, Chrestensen CA, Nyce M, Harp MG, Pelo JW, Cominelli F, Ernst PB, Pizarro TT, Sturgill TW, Worthington MT. MNK kinases regulate multiple TLR pathways and innate proinflammatory cytokines in macrophages. American journal of physiology. Gastrointest Liver Physiol. 2008;294(2):G452–9. https://doi.org/10.1152/ajpgi.00077.2007.
Scheper GC, van Kollenburg B, Hu J, Luo Y, Goss DJ, Proud CG. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J Biol Chem. 2002;277(5):3303–9.
Shiers S, Mwirigi J, Pradhan G, Kume M, Black B, Barragan-Iglesias P, Moy JK, Dussor G, Pancrazio JJ, Kroener S, Price TJ. Reversal of peripheral nerve injury-induced neuropathic pain and cognitive dysfunction via genetic and tomivosertib targeting of MNK. Neuropsychopharmacology. 2020;45(3):524–533. https://doi.org/10.1038/s41386-019-0537-y.
Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485(7396):109–13.
Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24(15):6539-49. https://doi.org/10.1128/MCB.24.15.6539-6549.2004.
Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93(1–2):139–48.
Acknowledgments
The authors would like to thank Han Saim Jeong and Aspen L. Samuel for the technical assistance.
Funding
This research was funded by the National Institutes of Health/National Institute of Neurological Disorders and Stroke, grant number NS096030 (M.D.B.), the University of Texas System STARS program research support grant (M.D.B.), the American Pain Society Future Leaders Grant (M.D.B.), and a Rita Allen Foundation Grant (M.D.B.).
Author information
Authors and Affiliations
Contributions
Conceptualization, N.L.S.; methodology, N.L.S.; analysis, N.L.S. and P.H.M.; data curation, N.L.S., L.B., and P.H.M.; writing manuscript and drawing figures, N.L.S., L.R.B., and P.H.M.; T.J.P. writing manuscript and collaborator; M.D.B participated and supervised in all aspects of the study conception to manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors except T.J.P. declare no conflict of interest. T.J.P. is a cofounder of 4E Therapeutics, a company developing MNK inhibitors for neuropathic pain. 4E Therapeutics played no role in funding the study.
Ethics approval
All procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Texas at Dallas Institutional Animal Care and Use Committee protocols 15-15 and 16-07.
Consent for publication
All authors have reviewed the contents of the manuscript being submitted and approve of its contents and validate the accuracy of the data.
Disclaimer
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Mody, P.H., Dos Santos, N.L., Barron, L.R. et al. eIF4E phosphorylation modulates pain and neuroinflammation in the aged. GeroScience 42, 1663–1674 (2020). https://doi.org/10.1007/s11357-020-00220-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00220-1