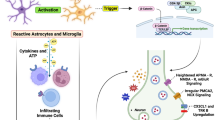
Abstract
Objective and design
Our aim was to determine an age-dependent role of Nav1.8 and ASIC3 in dorsal root ganglion (DRG) neurons in a rat pre-clinical model of long-term inflammatory pain.
Methods
We compared 6 and 24 months-old female Wistar rats after cutaneous inflammation. We used behavioral pain assessments over time, qPCR, quantitative immunohistochemistry, selective pharmacological manipulation, ELISA and in vitro treatment with cytokines.
Results
Older rats exhibited delayed recovery from mechanical allodynia and earlier onset of spontaneous pain than younger rats after inflammation. Moreover, the expression patterns of Nav1.8 and ASIC3 were time and age-dependent and ASIC3 levels remained elevated only in aged rats. In vivo, selective blockade of Nav1.8 with A803467 or of ASIC3 with APETx2 alleviated mechanical and cold allodynia and also spontaneous pain in both age groups with slightly different potency. Furthermore, in vitro IL-1β up-regulated Nav1.8 expression in DRG neurons cultured from young but not old rats. We also found that while TNF-α up-regulated ASIC3 expression in both age groups, IL-6 and IL-1β had this effect only on young and aged neurons, respectively.
Conclusion
Inflammation-associated mechanical allodynia and spontaneous pain in the elderly can be more effectively treated by inhibiting ASIC3 than Nav1.8.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from authors upon reasonable request.
References
Amir R, Argoff CE, Bennett GJ, Cummins TR, Durieux ME, Gerner P, Gold MS, Porreca F, Strichartz GR. The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain. 2006;7(5 Suppl 3):S1-29.
Stemkowski PL, Smith PA. Sensory neurons, ion channels, inflammation and the onset of neuropathic pain. Can J Neurol Sci. 2012;39(4):416–35.
Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13(7):533–48.
Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–84.
Ghlichloo I, Gerriets V. Nonsteroidal anti-inflammatory drugs (NSAIDs). 2023 May 1. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 31613522.
Reid MC, Eccleston C, Pillemer K. Management of chronic pain in older adults. BMJ. 2015;350:h532.
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–90.
Messina DN, Peralta ED, Seltzer AM, Patterson SI, Acosta CG. Age-dependent and modality-specific changes in the phenotypic markers Nav1.8, ASIC3, P2X3 and TRPM8 in male rat primary sensory neurons during healthy aging. Biogerontology. 2023;24(1):111–36.
Fang X, Djouhri L, McMullan S, Berry C, Okuse K, Waxman SG, Lawson SN. trkA is expressed in nociceptive neurons and influences electrophysiological properties via Nav1.8 expression in rapidly conducting nociceptors. J Neurosci. 2005;25(19):4868–78.
Hameed S. Na(v)1.7 and Na(v)1.8: Role in the pathophysiology of pain. Mol Pain. 2019;15:1744806919858801.
Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108(3):237–47.
Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci U S A. 1996;93(3):1108–12.
Cummins TR, Black JA, Dib-Hajj SD, Waxman SG. Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons. J Neurosci. 2000;20(23):8754–61.
Fjell J, Cummins TR, Dib-Hajj SD, Fried K, Black JA, Waxman SG. Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons. Brain ResMolBrain Res. 1999;67(2):267–82.
Kerr BJ, Souslova V, McMahon SB, Wood JN. A role for the TTX-resistant sodium channel Nav 18 in NGF-induced hyperalgesia, but not neuropathic pain. NeuroReport. 2001;12(14):3077–80.
Waldmann R, Champigny G, Lingueglia E, De Weille JR, Heurteaux C, Lazdunski M. H(+)-gated cation channels. Ann N Y Acad Sci. 1999;868:67–76.
Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;27(22):3047–55.
Steingrimsdottir OA, Landmark T, Macfarlane GJ, Nielsen CS. Defining chronic pain in epidemiological studies: a systematic review and meta-analysis. Pain. 2017;158(11):2092–107.
Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ. 2016;7:34.
Levy DR, Hunter N, Lin S, Robinson EM, Gillis W, Conlin EB, Anyoha R, Shansky RM, Datta SR. Mouse spontaneous behavior reflects individual variation rather than estrous state. Curr Biol. 2023;33:1358.
Shansky RM, Murphy AZ. Considering sex as a biological variable will require a global shift in science culture. Nat Neurosci. 2021;24(4):457–64.
Benitez SG, Seltzer AM, Messina DN, Foscolo MR, Patterson SI, Acosta CG. Cutaneous inflammation differentially regulates the expression and function of angiotensin-II types 1 and 2 receptors in rat primary sensory neurons. J Neurochem. 2020;152(6):675–96.
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63.
Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59(3):369–76.
Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27(50):13680–90.
Marsh B, Acosta C, Djouhri L, Lawson SN. Leak K(+) channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. MolCell Neurosci. 2012;49(3):375–86.
Schoor O, Weinschenk T, Hennenlotter J, Corvin S, Stenzl A, Rammensee HG, Stevanovic S. Moderate degradation does not preclude microarray analysis of small amounts of RNA. Biotechniques. 2003;35(6):1192–6.
Bustin SA. Why the need for qPCR publication guidelines?—The case for MIQE. Methods. 2010;50(4):217–26.
Siqueira SR, Alves B, Malpartida HM, Teixeira MJ, Siqueira JT. Abnormal expression of voltage-gated sodium channels Nav1.7, Nav1.3 and Nav1.8 in trigeminal neuralgia. Neuroscience. 2009;164(2):573–7.
Deval E, Noel J, Gasull X, Delaunay A, Alloui A, Friend V, Eschalier A, Lazdunski M, Lingueglia E. Acid-sensing ion channels in postoperative pain. J Neurosci. 2011;31(16):6059–66.
Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25(4):386–401.
Molliver DC, Lindsay J, Albers KM, Davis BM. Overexpression of NGF or GDNF alters transcriptional plasticity evoked by inflammation. Pain. 2005;113(3):277–84.
Pezet S, Krzyzanowska A, Wong LF, Grist J, Mazarakis ND, Georgievska B, McMahon SB. Reversal of neurochemical changes and pain-related behavior in a model of neuropathic pain using modified lentiviral vectors expressing GDNF. Mol Ther. 2006;13(6):1101–9.
Acosta C, Djouhri L, Watkins R, Berry C, Bromage K, Lawson SN. TREK2 expressed selectively in IB4-binding C-fiber nociceptors hyperpolarizes their membrane potentials and limits spontaneous pain. JNeurosci. 2014;34(4):1494–509.
Messina DN, Peralta ED, Acosta CG. Glial-derived neurotrophic factor regulates the expression of TREK2 in rat primary sensory neurons leading to attenuation of axotomy-induced neuropathic pain. Exp Neurol. 2022;357:114190.
Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-Adelta- or Aalpha/beta-fibres. ExpPhysiol. 2002;87(2):239–44.
Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K, Lawson SN. Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J Neurosci. 2006;26(27):7281–92.
Gao LL, McMullan S, Djouhri L, Acosta C, Harper AA, Lawson SN. Expression and properties of hyperpolarization-activated current in rat dorsal root ganglion neurons with known sensory function. J Physiol. 2012;590(19):4691–705.
Haskins W, Benitez S, Mercado JM, Acosta CG. Cutaneous inflammation regulates THIK1 expression in small C-like nociceptor dorsal root ganglion neurons. Mol Cell Neurosci. 2017;83:13–26.
Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, Kort M, Carroll W, Marron B, Atkinson R, Thomas J, Liu D, Krambis M, Liu Y, McGaraughty S, Chu K, Roeloffs R, Zhong C, Mikusa JP, Hernandez G, Gauvin D, Wade C, Zhu C, Pai M, Scanio M, Shi L, Drizin I, Gregg R, Matulenko M, Hakeem A, Gross M, Johnson M, Marsh K, Wagoner PK, Sullivan JP, Faltynek CR, Krafte DS. A-803467, a potent and selective Nav18 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci U S A. 2007;104(20):8520–5.
Huang X, Jin X, Huang G, Huang J, Wu T, Li Z, Chen J, Kong F, Pan X, Yan N. Structural basis for high-voltage activation and subtype-specific inhibition of human Na(v)18. Proc Natl Acad Sci U S A. 2022;119(30):2208211119.
McGaraughty S, Chu KL, Scanio MJ, Kort ME, Faltynek CR, Jarvis MF. A selective Nav18 sodium channel blocker, A-803467 [5-(4-chlorophenyl-N-(3,5-dimethoxyphenyl)furan-2-carboxamide], attenuates spinal neuronal activity in neuropathic rats. J Pharmacol Exp Ther. 2008;324(3):1204–11.
Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ, Cook SP, Kane S, Urban MO. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol. 2010;161(4):950–60.
Robson LG, Dyall SC, Sidloff D, Michael-Titus AT. Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurones throughout development and in aged animals. Neurobiol Aging. 2010;31(4):678–87.
Rafiyan M, Sadeghmousavi S, Akbarzadeh M, Rezaei N. Experimental animal models of chronic inflammation. Curr Res Immunol. 2023;4:100063.
Blanchard MG, Rash LD, Kellenberger S. Inhibition of voltage-gated Na(+) currents in sensory neurones by the sea anemone toxin APETx2. Br J Pharmacol. 2012;165(7):2167–77.
Verkest C, Salinas M, Diochot S, Deval E, Lingueglia E, Baron A. Mechanisms of action of the peptide toxins targeting human and rodent acid-sensing ion channels and relevance to their in vivo analgesic effects. Toxins (Basel). 2022;14(10):709.
Almarestani L, Fitzcharles MA, Bennett GJ, Ribeiro-da-Silva A. Imaging studies in Freund’s complete adjuvant model of regional polyarthritis, a model suitable for the study of pain mechanisms, in the rat. Arthritis Rheum. 2011;63(6):1573–81.
Ferdousi MI, Calcagno P, Sanchez C, Smith KL, Kelly JP, Roche M, Finn DP. Characterization of pain-, anxiety-, and cognition-related behaviors in the complete Freund’s adjuvant model of chronic inflammatory pain in Wistar-Kyoto rats. Front Pain Res (Lausanne). 2023;4:1131069.
Martin KK, Parvin S, Garraway SM. Peripheral inflammation accelerates the onset of mechanical hypersensitivity after spinal cord injury and engages tumor necrosis factor alpha signaling mechanisms. J Neurotrauma. 2019;36(12):2000–10.
Sendama W. The effect of ageing on the resolution of inflammation. Ageing Res Rev. 2020;57:101000.
Noh ASM, Chuan TD, Khir NAM, Zin AAM, Ghazali AK, Long I, Ab Aziz CB, Ismail CAN. Effects of different doses of complete Freund’s adjuvant on nociceptive behaviour and inflammatory parameters in polyarthritic rat model mimicking rheumatoid arthritis. PLoS ONE. 2021;16(12):e0260423.
Morgan D, Mitzelfelt JD, Koerper LM, Carter CS. Effects of morphine on thermal sensitivity in adult and aged rats. J Gerontol A Biol Sci Med Sci. 2012;67(7):705–13.
Thapa D, Barrett B, Argunhan F, Brain SD. Influence of cold-TRP receptors on cold-influenced behaviour. Pharmaceuticals (Basel). 2021;15(1):42.
Kabadi R, Kouya F, Cohen HW, Banik RK. Spontaneous pain-like behaviors are more sensitive to morphine and buprenorphine than mechanically evoked behaviors in a rat model of acute postoperative pain. Anesth Analg. 2015;120(2):472–8.
Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede DR. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150(3):439–50.
Ma L, Liu S, Yi M, Wan Y. Spontaneous pain as a challenge of research and management in chronic pain. Med Rev. 2022;2(3):308–19.
Strickland IT, Martindale JC, Woodhams PL, Reeve AJ, Chessell IP, McQueen DS. Changes in the expression of NaV17, NaV18 and NaV19 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur J Pain. 2008;12(5):564–72.
Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain. 2008;137(3):662–9.
Izumi M, Ikeuchi M, Ji Q, Tani T. Local ASIC3 modulates pain and disease progression in a rat model of osteoarthritis. J Biomed Sci. 2012;19(1):77.
Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32(6):1071–83.
Zhou RP, Wu XS, Wang ZS, Xie YY, Ge JF, Chen FH. Novel insights into acid-sensing ion channels: implications for degenerative diseases. Aging Dis. 2016;7(4):491–501.
Chen WH, Hsieh CL, Huang CP, Lin TJ, Tzen JT, Ho TY, Lin YW. Acid-sensing ion channel 3 mediates peripheral anti-hyperalgesia effects of acupuncture in mice inflammatory pain. J Biomed Sci. 2011;18(1):82.
Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21(20):8026–33.
Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury. J Pain. 2010;11(3):210–8.
Ding J, Zhang R, Li H, Ji Q, Cheng X, Thorne RF, Hondermarck H, Liu X, Shen C. ASIC1 and ASIC3 mediate cellular senescence of human nucleus pulposus mesenchymal stem cells during intervertebral disc degeneration. Aging (Albany NY). 2021;13(7):10703–23.
Dulai JS, Smith ESJ, Rahman T. Acid-sensing ion channel 3: an analgesic target. Channels (Austin). 2021;15(1):94–127.
Morgan M, Thai J, Trinh P, Habib M, Effendi KN, Ivanusic JJ. ASIC3 inhibition modulates inflammation-induced changes in the activity and sensitivity of Adelta and C fiber sensory neurons that innervate bone. Mol Pain. 2020;16:1744806920975950.
Atmaramani RR, Black BJ, de la Pena JB, Campbell ZT, Pancrazio JJ. Conserved Expression of Nav1.7 and Nav1.8 contribute to the spontaneous and thermally evoked excitability in IL-6 and NGF-sensitized adult dorsal root ganglion neurons in vitro. Bioengineering (Basel). 2020;7(2):44.
Kung CC, Huang YC, Hung TY, Teng CY, Lee TY, Sun WH. Deletion of acid-sensing ion channel 3 relieves the late phase of neuropathic pain by preventing neuron degeneration and promoting neuron repair. Cells. 2020;9(11):2355.
Lee JYP, Saez NJ, Cristofori-Armstrong B, Anangi R, King GF, Smith MT, Rash LD. Inhibition of acid-sensing ion channels by diminazene and APETx2 evoke partial and highly variable antihyperalgesia in a rat model of inflammatory pain. Br J Pharmacol. 2018;175(12):2204–18.
Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447(7146):855–8.
Luiz AP, MacDonald DI, Santana-Varela S, Millet Q, Sikandar S, Wood JN, Emery EC. Cold sensing by Na(V)18-positive and Na(V)18-negative sensory neurons. Proc Natl Acad Sci U S A. 2019;116(9):3811–6.
Askwith CC, Benson CJ, Welsh MJ, Snyder PM. DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature. Proc Natl Acad Sci U S A. 2001;98(11):6459–63.
Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321(5889):702–5.
Roza C, Laird JM, Souslova V, Wood JN, Cervero F. The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. J Physiol. 2003;550(Pt 3):921–6.
Kleggetveit IP, Namer B, Schmidt R, Helas T, Ruckel M, Orstavik K, Schmelz M, Jorum E. High spontaneous activity of C-nociceptors in painful polyneuropathy. Pain. 2012;153(10):2040–7.
Lee CH, Chen CC. Roles of ASICs in nociception and proprioception. Adv Exp Med Biol. 2018;1099:37–47.
DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90(1–2):1–6.
Zhang T, Zhang N, Zhang R, Zhao W, Chen Y, Wang Z, Xu B, Zhang M, Shi X, Zhang Q, Guo Y, Xiao J, Chen D, Fang Q. Preemptive intrathecal administration of endomorphins relieves inflammatory pain in male mice via inhibition of p38 MAPK signaling and regulation of inflammatory cytokines. J Neuroinflammation. 2018;15(1):320.
Cook AD, Christensen AD, Tewari D, McMahon SB, Hamilton JA. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018;39(3):240–55.
Matsuda M, Huh Y, Ji RR. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth. 2019;33(1):131–9.
Ngwainmbi J, De DD, Smith TH, El-Hage N, Fitting S, Kang M, Dewey WL, Hauser KF, Akbarali HI. Effects of HIV-1 Tat on enteric neuropathogenesis. J Neurosci. 2014;34(43):14243–51.
Chen X, Pang RP, Shen KF, Zimmermann M, Xin WJ, Li YY, Liu XG. TNF-alpha enhances the currents of voltage gated sodium channels in uninjured dorsal root ganglion neurons following motor nerve injury. Exp Neurol. 2011;227(2):279–86.
Gong W, Kolker SJ, Usachev Y, Walder RY, Boyle DL, Firestein GS, Sluka KA. Acid-sensing ion channel 3 decreases phosphorylation of extracellular signal-regulated kinases and induces synoviocyte cell death by increasing intracellular calcium. Arthritis Res Ther. 2014;16(3):R121.
Ross JL, Queme LF, Cohen ER, Green KJ, Lu P, Shank AT, An S, Hudgins RC, Jankowski MP. Muscle IL1beta drives ischemic myalgia via ASIC3-mediated sensory neuron sensitization. J Neurosci. 2016;36(26):6857–71.
Zhou R, Wu X, Wang Z, Ge J, Chen F. Interleukin-6 enhances acid-induced apoptosis via upregulating acid-sensing ion channel 1a expression and function in rat articular chondrocytes. Int Immunopharmacol. 2015;29(2):748–60.
de Magalhaes SF, Manzo LP, de Faria FM, de Oliveira-Fusaro MC, Nishijima CM, Vieira WF, Bonet IJM, Dos Santos GG, Tambeli CH, Parada CA. Inflammatory pain in peripheral tissue depends on the activation of the TNF-alpha type 1 receptor in the primary afferent neuron. Eur J Neurosci. 2021;53(2):376–89.
Wei S, Qiu CY, Jin Y, Liu TT, Hu WP. TNF-alpha acutely enhances acid-sensing ion channel currents in rat dorsal root ganglion neurons via a p38 MAPK pathway. J Neuroinflammation. 2021;18(1):92.
Acknowledgements
DNM and EDP: were supported by doctoral fellowships from the National Research Council of Argentina (CONICET). CGA is a Staff Scientist of the same organization. This work was funded by Fondo Nacional para la Ciencia y la Tecnología (FONCyT-ANPCyT PICT-2019-02666 to CGA), Proyecto de Financiamiento de Unidades Ejecutoras (PUE-2017-0025 to CGA) and by the Proyectos de Investigación Plurianual 2021–2023 (PIP-2230 to CGA). The sponsors of this research were not involved in the experimental design, writing of the manuscript or the decision to submit it. We thank Sean Patterson (IHEM-CONICET) for critical reading and editing of the manuscript.
Funding
Consejo Nacional de Investigaciones Científicas y Técnicas, Studentships, Studentships, CIC, Permanent Staff
Author information
Authors and Affiliations
Contributions
Conceptualization: D.N.M & C.G.A.; Methodology: D.N.M., E.D.P. & C.G.A.; Formal analysis and investigation: D.N.M., E.D.P. & C.G.A.; Writing—original draft preparation: D.N.M. & C.G.A.; Writing—review and editing: D.N.M., E.D.P. & C.G.A.; Funding acquisition: C.G.A.; Supervision: C.G.A.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Messina, D.N., Peralta, E.D. & Acosta, C.G. Complex alterations in inflammatory pain and analgesic sensitivity in young and ageing female rats: involvement of ASIC3 and Nav1.8 in primary sensory neurons. Inflamm. Res. 73, 669–691 (2024). https://doi.org/10.1007/s00011-024-01862-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-024-01862-z