Abstract
The purpose of this review was to survey the recent applications of the diffusive gradients in thin films (DGT) technique in the assessment of mobility and bioavailability of nutrients and potentially toxic elements (PTEs) in agricultural soil. Many studies compared the capabilities of the DGT technique with those of classical soil chemical extractants used in single or sequential procedures to predict nutrients and PTE bioavailability to crops. In most of the published works, the DGT technique was reported to be superior to the conventional chemical extraction and fractionation methods in obtaining significant correlations with the metals and metalloids accumulated in crops. In the domain of nutrient bioavailability assessment, DGT-based studies focused mainly on phosphorous and selenium labile fraction measurement, but potassium, manganese, and nitrogen were also studied using the DGT tool. Different DGT configurations are reported, using binding and diffusive layers specific for certain analytes (Hg, P, and Se) or gels with wider applicability, such as Chelex-based binding gels for metal cations and ferrihydrite-based hydrogels for oxyanions. Overall, the literature demonstrates that the DGT technique is relevant for the evaluation of metal and nutrient bioavailability to crops, due to its capacity to mimic the plant root uptake process, which justifies future improvement efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the growing of the world’s population, the demand for food is continuously increasing; thus, the agricultural sector goes to intensive farming. This entails the continuous cultivation of crops on soil using a substantial application of fertilizers and pesticides (Chen et al. 2023). Besides the benefits, this may have a negative effect on the environment, due to both the release of the nutrients added in excess and to the soil contamination with potentially toxic elements (PTEs) (Kuziemska et al. 2023). In addition, the development of industrial activities resulted in the release of PTEs in all environmental compartments, including the agricultural soil (Park et al. 2023). PTEs may have extremely harmful effects on the ecosystem, affecting the plant growth and being transferred to the food chain, causing human health risks (Dippong et al. 2022; Zulkernain et al. 2023; Jiang et al. 2023). There are many PTE species that were found in the soil in increased concentrations (Roba et al. 2016; Petrean et al. 2023). Some of the PTEs, like Fe, Zn, Co, Cu, Cr, B, and Mn, are also known as micronutrients for crops, small amounts of these elements being required for plant development. At low concentrations, these PTEs can help some cellular functions in plants, as well as pigment biosynthesis, enzyme activities, photosynthesis, sugar metabolism, respiration, nitrogen fixation, and other functions, but even these elements become toxic at increased concentrations, affecting plant growth (Rashid et al. 2023). Other PTEs such as Cd, Pb, As, Hg, and Sb are non-essential and highly toxic even at low concentrations and affect biota, and finally human health (Briffa et al. 2020; Senila et al. 2022; Thalassinos et al. 2023).
Consequently, the prediction of the nutrient and PTE transfer from soil to the crops has become an increasing concern worldwide (Ning et al. 2021; Gao et al. 2022a, b; Zhou et al. 2023). The key process in the prediction of nutrient and PTE transfer through the food chain is the assessment of their availability in soil for improving the fertilization efficacy, while reducing the soil contamination (Wenzel et al. 2022), but also for preventing the food web contamination through the transfer to crops above the safe limits. Thus, assessing the bioavailability of PTEs and nutrients in agricultural soils has critical implications for food security and for a sustainable agriculture.
Many studies on nutrients and PTEs in soil were based on the measurement of their total concentration, even though their available concentrations would have been more informative. Moreover, in general, the studies on nutrient and PTE availability in soil were carried out by using sequential or single-chemical extraction procedures, but these offer only records of diverse fractions of elements in the soil (Senila 2014; Gao et al. 2022a, b). The diffusive gradients in thin film (DGT) technique was well documented as being a superior tool for the evaluation of metal and nutrient bioavailability to biota due to its capacity to mimic the uptake of elements by the plant roots (Tandy et al. 2011; Zhang and Davison 2015; Marrugo-Madrid et al. 2021). When deployed into the soil, the DGT device introduces a sink for free ions in the soil solution, generating a diffusive flux of those ions into the DGT probe. As the free ions are gradually uptaken by the DGT device, their concentration in the adjacent soil solution decreases causing a disequilibrium among the free ions in the soil solution, their complexes, and their forms fixed onto soil solid phases. In response to the removal of the free ions, labile complexes dissociate, and their forms bound to the soil solid phase can also desorb depending on their availability, being thus resupplied to the soil solution. This uptake mechanism is similar to the plant root uptake into their rhizosphere (Davison and Zhang 2012; Letho 2016). Generally, DGT measurements are mostly independent of the soil characteristics, which could make DGT a good practice for advancing soil quality standards (Tian et al. 2018).
Since several previous review papers (Zhang and Davison 2015; Santner et al. 2015; Marrugo-Madrid et al. 2021; Wei et al. 2022; Guan et al. 2022) and the book Diffusive gradients in thin-films for environmental measurements (Davison 2016) presented the concept and theory of DGT, it was the aim of this review to provide an update of the existing DGT knowledge for scientists and practitioners, related to the application of this technique to the agricultural field, for assessing the nutrient and PTE bioavailability in soil. Although the principles of DGT technique were not significantly changed since it was first reported until today, this review aims to provide new trends and findings from its application, or new binding gels developed for various analytes. Since the DGT was found to be generally superior to other techniques in predicting element transfer to crops, this review brings together the newest advancements of the DGT uses on agricultural soil emphasizing the necessity and importance of ensuring food security. The literature published in the last 5 years was mainly analyzed to observe the trend, but several previously published reference works for the DGT development were also considered.
Methodology to collect data
The search keyword “DGT agricultural soil” was used for the Web of Science Core Collection database, and n = 276 documents were found, of which 274 articles (including 3 proceeding papers and 2 review articles) and 2 book chapters. The 143 articles published between 2019 and 2023 were selected and were examined to involve agricultural soil and/or crops. Those dealing with the use of the DGT technique for assessing the bioavailability of metals and nutrients from agricultural soil were included in this literature review.
DGT applicability for element availability in soil, DGT procedure, and deployment in soil
Theory of DGT measurement for nutrients/PTEs in soil
An overview of the applicability of DGT in agricultural soil is presented in Fig. 1. The DGT technique was created in 1994 by Hao Zhang and William Davison for the determination of trace metals in water (Davison and Zhang 1994). The functioning principle of the DGT technique was widely presented by Davison and Zhang (2016). Here, we are briefly presenting the procedure used for estimating the fractions from soils which are bioavailable to crops.
The DGT technique is founded on Fick’s first law of diffusion, which relates the diffusive flux of analyte to the gradient of the concentration. This flux is controlled by a diffusive layer with a known thickness (Δg) which is placed between the analyzed soil solution and a resin gel that accumulates the analytes (Zhang and Davison 1995). The analyte concentration in the analyzed soil solution can be calculated using Eq. (1) (Zhang and Davison 1995):
where M is the mass of metal bound by the resin gel, Δg is the diffusive layer thickness (sum of the diffusive gel and filter paper thickness), t is the time for deployment (in seconds), D is the diffusion coefficient through the diffusion layer, and A is the exposed area to the soil solution.
The mass of analyte accumulated on the resin gel may be eluted from the gel using an appropriate volume of eluents (Ve). The measured concentration of the analyte in the eluent (Ce), the elution factor (fe), and the volume of the resin gel (Vg) are used to calculate M using Eq. (2):
DGT procedure and deployment in soil
The procedure of using DGT devices into the soil for passive sampling of labile fractions of analytes is based on their deployment saturated with water or at the maximum water holding capacity (MWHC). Other authors used water until 80% of soil MWHC (Babalola and Zhang 2021). The collected soil samples air dried and sieved ≤ 2 mm are mixed with deionized water to MWHC to form a slurry (www.dgtresearch.com). The quantity of soil used is not a very important aspect for typical DGT placement times (24 h). However, a depth of soil adjacent to the DGT sampling device needs to be of at least 1 cm for a deployment period of 24 h considering the depletion of analyte in that area. Typical quantities of soil used per DGT device are of about 20–200 g (Jolley et al. 2016). The soil-water mixture is left for 24 h to equilibrate, while being covered in order to prevent evaporation. It is recommended to perform the DGT deployments under controlled temperature conditions, since this is an important parameter that influences the diffusion coefficients. Deployment periods may vary, but usually they are of about 24 h, which is enough to provide a quantification of the elements (Jolley et al. 2016). A scheme showing the main steps of DGT procedure for the assessment of DGT-labile fraction of elements in soil is presented in Fig. 2.
The analytes retained in the resin gels are usually extracted (using different eluents depending on the analyte types), then analyzed by appropriate techniques, among the most widely used: atomic absorption spectrometry with flame of graphite furnace atomization (FAAS or GFAAS), inductively coupled plasma optical emission spectrometry (ICP-OES), anodic stripping voltammetry (ASV), inductively coupled plasma mass spectrometry (ICP-MS), and hyphenated techniques based on inductively coupled plasma mass spectrometry and liquid chromatography for speciation (LC-ICP-MS). Another possibility is to analyze the gel directly, without a dilution step, using laser ablation ICP-MS (LA-ICP-MS), proton-induced X-ray emissions (PIXE), X-ray fluorescence spectroscopy (XRF) (Wei et al. 2022), or thermal desorption atomic absorption spectrometry (TDAAS)—for Hg analysis (Senila et al. 2023). Also, imaging tools using a DGT gel combined with planar optode (PO) can be used on root systems for high-resolution imaging of solute distribution in porewaters (Santner et al. 2015; Smolders et al. 2020).
Aspects regarding the DGT use in prediction of nutrient/PTE bioavailability
The bioavailability of a certain chemical substance in soil refers to its freely available fraction, not sorbed or sequestered on soil particles, which is mobile or easily mobilizable, and at which the biota is most exposed (Guan 2019). Meanwhile, bioavailability processes were defined by the National Research Council Committee on Bioavailability of Contaminants in Soils and Sediments as “the individual physical, chemical, and biological interactions that determine the exposure of plants and animals to chemicals associated with soils and sediments” (NRC 2003).
A DGT device deployed the soil slurry represents a sink for analyte labile species in the soil solution. Labile species primarily include the free ion and simple inorganic complexes which dissociate fast enough and have diffusion coefficient values close to that of free ions (Davison and Zhang 2012). Most trace elements, except for Hg, have inorganic complexes that are fully labile within the period of DGT deployment (Letho 2016). Some organic complexes whose sizes are in the colloidal range can also pass through the diffusive gels, but under usual deployment period (hours to days), only limited quantities of colloids pass through the diffusive gels because of their much lower diffusion coefficients (Gao et al. 2019).
When DGT device is deployed in the soil slurry, there may be a considerable depletion of the analyte concentration in the soil solution from the soil adjacent the interface between soil and DGT. To compensate the DGT-induced depletion, elements that are in readily available fraction in the solid phase are re-supplied from the soil solid phase to the solution, contributing to DGT-measured concentration (Degryse et al. 2009; Guan 2019). Consequently, DGT-labile concentration (CDGT) of an element integrates soil properties into only one key parameter (Guan 2019). This aspect is highly important and represents a major advantage of the DGT measurement over the “classical” chemical extraction methods, which do not account the soil properties.
The ratio R of CDGT to the measured concentration of a specific element in soil solution (csoln) is a parameter which displays the soil’s capability to supply solute to the soil solution after the depletion at the DGT-soil interface:
For a usual deployment in of DGT devices in soil slurry of 24 h, and a frequently used diffusion layer thickness (0.093 cm), there are three types of solute supply to the DGT (Zhang et al. 1998): (i) sustained case (R > 0.8), which means a continuous and rapid supply of solute from the soil particles to sustain the flux into the DGT; (ii) partially sustained case (0.2 ≤ R ≤ 0.8), or intermediate case, when supply of solute forming the soil solid phase exists, but is insufficient to sustain the maximum flux into to DGT; (iii) diffusive case, or unstained case (R < 0.2), when the supply for solid phase is extremely low or is no resupply of solutes (Letho 2016).
For the partially sustained case, in which there is a diffusional supply from both the soil solution and release from the solid phase, the interfacial concentration can be associated with the effective concentration of labile species, CE, a concept developed by Zhang et al. (2001). CE signifies the supply of analyte to any sink, DGT or a crop, that originates from diffusion from two above-mentioned sources. Because only the diffusion concentration is considered, which is a similar process to those occurred in the rhizosphere of plants, CE can be linked to the uptake of the plant (Marrugo-Madrid et al. 2021). CE can be calculated as the ratio between CDGT and Rdiff, in which Rdiff represents the ratio of the time-averaged concentration at the DGT interface to the concentration in the soil solution for diffusion case only.
R diff can be computed using the numerical model 2D-DIFS (two-dimensional DGT induced fluxes in sediments) adapted by Sochaczewski et al. (2007) from the 1D-DIFS software developed by Harper et al. (2000). The DIFS program enables the calculation of the ratio of DGT concentration to total solution concentration, which also depends on the adsorption/desorption kinetics of elements within the soil. The distribution of the labile element fraction between the soil solid and the pore water concentration is measured by the distribution coefficient, Kdl (cm3 g−1), while for the response time to depletion the parameter Tc (s) is employed (Guan et al. 2017). To calculate Rdiff only for the diffusive case, a very large value for Tc or a value for Kdl close to zero should be used. Thus, the DIFS program can be used to simulate Rdiff, Kdl, and Tc (Sochaczewski et al. 2007).
It should be noted that, from the first investigation on DGT as a surrogate for estimating plant uptake of several elements (Cd, Co, Cu, Ni, Pb, and Zn) carried out by Davison et al. (1999) to many other studies reported until present, in general, DGT provided element concentrations better correlated with those accumulated by plants. However, DGT does not always provide the best nutrient/metal bioavailability prediction. This is explained by the fact that bioavailability depends not only on the speciation in soil but also on the receptor (plant characteristics), which DGT cannot account for. For example, plant roots limit toxic metal uptake at toxic concentrations. Thus, the supply from the soil is not totally accumulated, and DGT measurements can overrate metal bioavailability. Also, plant exudates may modify elements’ solubility and mobility in the rhizosphere area (Guan et al. 2022). Thus, as much as possible, the analyzed soil substrate should be carefully chosen in the rhizosphere zone. It should also be considered that the concentration of metals/nutrients in the shoots depends on their own translocation factor (Guan et al. 2022). Other reasons for differences between DGT-predicted bioavailability and accumulation by plants include the higher moisture content in soil during the DGT deployment compared to that during plant growth, which determines higher diffusion fluxes, differences between the period of DGT deployment (usually 24 h) and period of plant growth (weeks), differences between DGT device and roots geometries (that have smaller radius), and presence of root hairs (Degryse et al. 2009; Degryse and Smolders 2016).
DGT has the potential to be deployed in situ, although this direction has not yet been extensively used until now due to potential issues related to the deficiency of parameter control, mainly regarding the soil moisture. Several reports on particular cases of DGT application to wet soils, such as rice fields, were published (Wang et al. 2021; Chen et al. 2022; Wang et al. 2022a).
DGT applications to nutrient labile fractions in soil
DGT applications to nutrients in agricultural soils deal with studies on phosphorous and selenium bioavailability, but nutrients such as potassium, manganese, and nitrogen forms were also considered in such studies. A total of 26 papers published in recent years have been identified and are discussed in this review. The main trends that can be observed related to the applications of the DGT technique for nutrients in these papers are the evaluation of bioavailability changes after the application of different amendments to soil and the use of DGT for the prediction of the uptake in different crops. The main findings from these researches are detailed below. The effects on P availability were tested after the addition of biochar (Chen et al. 2022; Yang and Lu 2022), or other organic and inorganic fertilizers containing P (Nobile et al. 2018; Kang et al. 2021; Wenzel et al. 2022; Zhang et al. 2023). In all cases, the DGT was reported as a useful tool in assessing the impact of amendments on P availability.
Chen et al. (2022) studied the fluxes of P in the rice rhizosphere using DGT, high-resolution dialysis (HR-Peeper), and zymography techniques. DGT measurements indicated that long-term biochar addition to the soil significantly diminished the diffusion and resupply capacity of P from the soil solid phase to the soil solution, thus lessening the risk of P release into the environment (Chen et al. 2022). Yang and Lu (2022) used a combination of chemical extraction and DGT technique to assess the phosphate availability of in straw/biochar-amended soils, and it was found that biochar augmented P availability and soil pH more than straw returning. Kang et al. (2021) conducted pot and field tests in drip-irrigated calcareous soil and used DGT next to other P analyses to assess changes in its availability in soil. With the aid of the DGT technique, the authors clarified that repetitively releasing P through the fertigation method is suggested as an efficient P application approach in drip-irrigated field. P in soil was also evaluated by the DGT technique and classic extractions in order to control the effects of soil type and fertilizer amendment on the P availability and to assess the ratios of inorganic P vs. organic P in soil (Nobile et al. 2018).
The DGT technique was used to quantify the availability of P in several types of sewage sludge-based fertilizers containing P fertilizers (Vogel et al. 2017). Combinations of fertilizer and soil were incubated for several weeks and DGT devices were immersed at different moments. It was found that plant-available P was obtained after 2 weeks of incubation. In a previous study, Vogel et al. (2021) combined DGT and 13P NMR to determine P species in soil. This combination allowed the authors to identify organic P species in solutions. DGT measurements were used to assess the wheat grain yield in long-term fertility experiments (Wenzel et al. 2022). DGT was reported to be superior to the classical quantity and intensity tests, due to its ability to mimic, like plant roots, P diffusion, and resupply from the soil solid fraction. A 5-year fertilization trial was employed to assess the influence on soil P fractionation (Zhang et al. 2023). The P resupply was simulated using DGT and DGT-induced fluxes in soils (DIFS), throughout the maize season, under five conditions of fertilization: no fertilizer, chemical fertilizer, chemical fertilizer joint with bone meal fertilizer, crop straw, and bioorganic fertilizer. With the aid of DGT and DIFS investigation tools, it was observed that organic fertilization, particularly NPKC and NPKM treatments, provided superior enrichment effects on the P supply pool and P resupply for improved plant P uptake.
In other papers, the DGT technique was used to assess P mobility in soils and its bioavailability to various agricultural crops. In general, P uptake by crops or P in soil solution was better correlated with P measured by DGT. P mobility in 75 topsoil samples from a plastic-covered greenhouse vegetable production form China was assessed, and it was found that DGT is a precise predictor of P mobility in soils, since DGT results for P provided a very strong correlation (r2 = 0.97) with P in soil solution, superior to that obtained by conventional soil extractable P test methods (sodium bicarbonate extractable—Olsen P, ammonium oxalate extractable P, MehlichIII P) (Kalkhajeh et al. 2018). Large-scale experiments were conducted to compare the soil tests for available P in agricultural soil from five countries across Europe (Nawara et al. 2017). P availability was evaluated with five tests: ammonium oxalate, ammonium lactate, Olsen P, CaCl2, and DGT, in 11 different soil types, cultivated with different crops. All five tests were positively correlated to the P accumulated by different crops. However, no test was considerably superior than the others, while the oxalate extraction had the weakest correlation. In a later study, Nawara et al. (2018) used the same methods to assess the P bioavailability in agricultural soil, but in depleting P speciation in pot experiments. A strong positive correlation (r2 = 0.73) was obtained for the P measured by DGT and plant uptake. Tuntrachanida et al. (2022) explored the fractionation and solubility of P in tropical agricultural soils, by combining the DGT technique to measure P fluxes in soil with spectroscopic measurements. With these techniques, the authors were able to find the association between P and different minerals in soil. The DGT concentration of P in the soil solution was extremely variable, with the maximum and the lowermost values detected in acidic and coarse soils, and in alkaline- and fine-textured soils, respectively. The DGT technique and calcium acetate lactate have been used to predict crops (winter wheat and spring barley) response in long-term P fertilization experiments. DGT was found to be superior in predicting P transfer to the two crops (r2 = 0.42 for barley, r2 = 0.32 for wheat grain yield, and r2 = 0.36 for wheat grains) (Hill et al. 2021). The DGT technique has been used to assess the bioavailability of P and other nutrient elements in flooded soils cultivated with rice crop. Wang et al. (2019a) studied, by means of DGT technique combined with high-resolution dialysis, the Fe and P interactions in flooded soils with rice crop. Using this combination of techniques, the authors observed a depletion of P and Fe(II) in porewaters around the rice root area. DGT was also used to evaluate P availability in agricultural soils of Scandinavia (Denmark, Sweden, Norway, and Finland) used in pot experiments for growing spring barley. In this case, DGT was also compared with three frequently used soil extraction methods: sodium-bicarbonate (Olsen P), ammonium lactate (PAL), and ammonium acetate (PAAC) (Mundus et al. 2017). Crops in pot experiments responded to fertilization and accumulated P, but there was a weak or no correlation with extractable P in soil, even with the DGT technique. Since in field conditions, extractable P measured in soils 30 days after establishment predicted P concentrations in leaves of unfertilized plants (r2 = 0.83), the authors emphasized the significance of pot size relative to the produced biomass in order to avoid the P depletion in areas surrounding the roots.
The DGT technique has also been developed to simultaneously determine the bioavailability of cationic nutrients (e.g., K) and P in agricultural soils by employing new types of retention gels. A resin gel has been created by combining amberlite and ferrihydrite for the simultaneous determinations of two important nutrients in soil, K and P (Zhang et al. 2013). It was showed that the developed gel has the capacity to quantify plant-available P and K in soils. In another study (Zhang et al. 2014), the DGT with the same binding phase was investigated to assess the influence of competing cations in solution on K uptake. The diffusion coefficient for K was lowered by the presence of competing cations. It was also reported that this mixed gel had the capability to quantify Ca and Mg. In a later study, this type of binding gel was used to estimate the necessities for K as fertilizer for wheat in a glasshouse trial (Zhang et al. 2017).
Selenium is an essential micronutrient, of which both deficiency and excess may have negative effect on biota. Thus, the studies of Se bioavailability using DGT in soil rise a high interest for researchers, reflected by eight recent papers dealing with Se bioavailability. DGT with ferrihydrite binding gel were mainly used for Se accumulation, and the results generally well predicted the Se uptake by crops such as Brassica juncea from fertilized soils, with correlation coefficients between 0.89 and 0.99 (Dinh et al. 2021), pak choi from soils amended with selenite and selenate, with correlation coefficients of 0.364 and 0.957 for shoots, and 0.909 and 0.876 for roots (Peng et al. 2019), purple cabbage, broccoli, wheat, and mustard from selenite or selenate-amended soils in pot experiments (Peng et al. 2017). Se bioavailability and accumulation in pak choi were evaluated by comparing the DGT technique with the chemical extraction methods (Peng et al. 2020). It was reported that the plant uptake of Se(IV) was better predicted by the DGT technique (r = 0.933 for shoots and r = 0.943 for roots) than by the chemical extraction methods, while the Se(VI) uptake was also well predicted by DGT (r = 0.885 for shoots and r = 0.841 for roots), but was better predicted by the KH2PO4–K2HPO4 extraction (r = 0.913 for shoots and r = 0.853 for roots). Wang et al. (2019b) reported on the use of the DGT technique and classical chemical extractions to evaluate the uptake of Se by the maize from naturally enriched soils. The authors found that the DGT can consistently predict the uptake of Se by maize (r = 0.933). Zhang et al. (2020) assessed Se bioavailability to Brassica juncea in soils, by using DGT and chemical extraction methods. The correlation coefficients between the Se transfer rates to Brassica juncea and the bioavailable Se content in soil, measured by different techniques, followed the trend DGT > KCl > water > EDTA> KH2PO4 > NaHCO3 extractions. Sequential chemical extractions and DGT were used to assess Se fractionation in pot experiments. It was found that the Se concentrations were generally resulting from soluble and exchangeable Se fractions (Lyu et al. 2021). However, in another study on soil amended with S and P, the Se content in soil measured by DGT was not significantly correlated with the Se content of pak choi. This may be explained by the fact that DGT cannot reflect the competitive relationship between P, S, and Se at the plant root uptake sites (Jiang et al. 2022).
Kodithuwakku et al. (2023) developed a DGT device for the determination of NO3–N and NH4–N in soil solutions. The used resin gel was either based on A520E (anion exchange resin) or on PrCH (cation exchange resin), while an agarose-type gel was used as diffusive layer, covered with a polyethersulfone filter membrane. The developed DGTs were able to measure low concentrations of NO3–N and NH4–N in soils. The concentrations assessed by DGT and by extraction in 2M KCl were significantly correlated for NO3–N (r2 = 0.53).
Table 1 presents selected works observing the use of DGT to assess nutrient mobility in soil and relations with different crops, presenting the analytes, used DGT tools, main experimental conditions, and main findings.
DGT applications for heavy metal labile fractions in soil
PTEs group includes heavy metals and several metalloids and are considered the main soil contaminants (Bai et al. 2023). Thus, they have received substantial consideration in recent studies. A total of 57 recent publications dealing with DGT in soil focused on heavy metals such as Cd, Pb, Zn, Cu, Cr, etc., while among the toxic metalloids, As was the most studied. In the studies involving metallic cations, the Chelex-type resin gel was predominantly used, while in the metalloids case, the ferrihydrite-type binding gel was mostly used. A particular attention was paid to Hg determination, due to its special characteristics and high toxicity. Numerous studies focused only on this element, probably because the DGT with Chelex binding gel, which is used for a majority of metallic cations, but is not suitable for the Hg determination. Consequently, specialized DGT tools for Hg and its species were developed and used.
Environmental studies using the DGT for assessing the PTE availability in soil and their transfer rate to crops
DGT has proven to be a beneficial technique for many studies dealing with the availability of PTEs in soil and their transfer to crops, within the 28 papers published in recent years on this topic. PTE measurement by DGT tends to be well correlated with crop uptake. Table 2 displays selected works observing the use of DGT to evaluate heavy metal mobility in soil and their transfer to different crops (maize, wheat, tomato, bean, rice, barley, turnips, eggplants, spinach, potatoes, onion, corn, peppermint, lettuce, garlic, parsley, carrot, pak choi). A tendency that can be observed in these studies is that the DGT is not yet used alone to assess metal bioavailability in soils; other “classical” chemical extractions are used for comparison purposes, demonstrating that DGT still needs to be validated. Another common finding is that in some papers, the DIFS software was used to calculate the element’s effective concentrations. However, other authors preferred to use the CDGT directly for correlations with crop uptake. While some studies deal with the multi-element determination of PTEs, other studies studied only a specific contaminant, such as Cd, As, or Zn.
Bai et al. (2023) correlated directly CDGT of PTEs (Cr, As, Cu, Zn, Cd, and Pb) from rhizosphere soil with the accumulation by maize and wheat. For comparison, the rhizosphere soil samples were analyzed for their PTE total content and extractible forms in soil solution and EDTA and DGT-labile fractions. Among the methods used in soil analysis, DGT was reported to provide, in general, more accurate results in simultaneously predicting PTE transfer to crops, especially for grains. Also, DGT bioavailability prediction was less impacted by soil pH than other extractions.
Galhardi et al. (2020) used sequential extraction (BCR), single extraction, and CDGT to assess the fractionation of As, Pb, Cd, Ni, Cu, Cr, Mn, Zn, Ba, U, REEs (La to Lu). The authors used DGT with Chelex resin gel to assess all available fractions of PTEs and REEs. They reported that chemical extractions and DGT correlated better with the metal bioavailability to corn, as compared to the total metal contents in soil. CDGT of PTE (Cd, Pb, Cu, Zn, Co, Cr, Mn, Ni, and Fe) and PTE concentration in soil solution were measured (Senila et al. 2024). A similar trend of soil resupply capacity (estimated by the R-ratio between the CDGT and the concentration in the soil solution) was observed with the bioaccumulation factors in Russula virescens mushroom. CDGT was also measured for Fe, Mn, Cd, and As labile fractions in flooded paddy soils (Wang et al. 2022b) or to study the effect of soil redox modifications on the activities of Cd and Cu in soil (Wang et al. 2023a). A new type of resin gel combined with layered double hydroxide nanoparticles changed with diethylenetriaminepentaacetic acid was created for the use of DGT tools to measure eight anions and cations in waters and soils (Wang et al. 2023b). A hyperaccumulator plant of Cd and Zn, Sedum plumbizincicola, was grown in a pot experiment (Zhou et al. 2019). Soil total Zn and water soluble, CaCl2-extractable, and CDGT Zn concentrations were measured and used to predict the shoot Zn concentration. Dissimilar to many other studies, in this study, CaCl2 extraction produced the strongest correlations with plant uptake than CDGT, results that were explained by two main reasons: Zn uptake by S. plumbizincicola was not limited to diffusion, while the behavior of hyperaccumulating species to increase metals solubility by the release of organic acids, which increase metal uptake. DIFS model was used to assess CE for As, Cr, Cu, Pb, and V in urban soil (Xu et al. 2019a). Cd bioavailability in soil was extensively studied both by CDGT as well as by estimating its CE using DIFS. Thus, DGT and DIFS were employed to evaluate the Cd bioavailable fraction and to predict its transfer to maize and its mobility in agricultural soils (Chen et al. 2021). Bioavailable Cd measured by DGT was significantly correlated with Cd accumulated in maize grains (r2 = 0.92).
C DGT also was found to predict well Cd transfer to crops. Thus, CDGT in agricultural soils was reported to be positively correlated (r2 = 0.95) with Cd uptake by pak choi in a greenhouse experiment (Dai et al. 2017), by cocoa bean (r2 = 0.5) (Gramlich et al. 2018), and turnips and eggplants grown in greenhouse vegetable production systems (r2 values from 0.53 to 0.70) (Tian et al. 2018). CDGT Cd was used to predict Cd uptake by the hyperaccumulator plant S. plumbizincicola in different agricultural soil categories. Using CDGT and piecewise equations, Cd uptake could be predicted at different intervals of soil properties (Wu et al. 2018). In a study on Cd uptake from agricultural soil to spinach leaves, potato tubers, onion bulbs, and wheat grain grown across New Zealand (Yi et al. 2020), the extraction in Ca(NO3)2 predicted more than 76% of the variability in the Cd concentrations in onion bulbs and spinach leaves, whereas CDGT and porewater Cd concentration better estimated the Cd transfer to potatoes and wheat grains.
C DGT in rhizosphere soil of several crops (rice, corn, peanut, and sweet potato) also showed good correlation with its accumulation in those crops (r2 in the range 0.64–0.90) (Guo et al. 2023). In a study on paddy soil, CDGT of Cd was correlated with Cd concentration in the rice grains, straws, or roots. CDGT showed a significant correlation with Cd in crop parts (r = 0.733 for grains, r = 0.833 for straw, and r = 0.680 for roots) (Li et al. 2018). In another study, a significant correlation (r = 0.818) was obtained between CDGT Cd in paddy soil and Cd in rice grains (Xiao et al. 2020). Soil chemical extraction and soil-plant transfer modeling methodologies were used to predict the Cd bioavailability to rice in a large-scale experiment on samples collected from 278 sites in the Guangxi province, China (Wen et al. 2020). CDGT Cd well estimated Cd in rice grains (r2 = 0.73), better than Cd in soil solution (r2 = 0.43).
Almendros et al. (2020) used the CDGT and low-molecular-weight organic acids (LMWOAs), CaCl2, DTPA-TEA, water, and NH4Ac chemical extractants to predict the Zn transfer to crops (tomato) from soils amended with ZnO nanoparticles in a greenhouse experiment. The Pearson correlation coefficients (r) between log-transformed values of Zn concentrations in crop and CDGT ranged between 0.787 and 0.915. Later, the same group of research reported CDGT for Zn well correlated with Zn accumulated by beetroot and green pea, with Pearson correlation coefficients between log-transformed values of the Zn concentrations in crops and CDGT in the range 0.78–0.96 (Almendros et al. 2022).
Another element in soil extensively studied by DGT was As. Ren et al. (2022) studied the absorption/desorption of As in farmland soils, together with soil P. Cerium oxide–based DGTs were used for the simultaneous determination of As and P in four different categories of agricultural soils in order to investigate As and P migration behaviors. It was reported that both P and As can attain the equilibrium of resupply in 0.7–18 min under DGT depletion. The lability of As and Sb in agricultural conditions in anthropogenic contaminated soils was assessed using various approaches: CDGT, soil solution extraction, and sequential extraction and accumulation by radish As and Sb contents in radish were significantly correlated (r2 = 0.97–0.99) with their accumulation in crop tissues (Ngo et al. 2016; Ngo et al. 2020). DGT combined with the high-resolution dialysis (HR-Peeper) technique was employed to obtain the distributions of soluble Fe(II), soluble reactive P, and labile P and Fe in the root area of rice (Wang et al. 2019a). DGT with 2.0-mm vertical resolution and hydrochemical monitoring were used to investigate the effects of flooding and flora on the Fe-redox and hydrochemical change in the soil porewater (Wu et al. 2021). It was found that the release of Fe(II) from the wetland rhizosphere due to flooding could have an effect on the release of Fe-associated metals from the riparian marshland to the surface water.
Studies using DGT as tool for the evaluation of the PTE mobility changes in soil remediation processes
The DGT technique has been used to assess the PTE mobility variations in soil remediation processes in 24 recent papers examined in this review. Among the studied topics, the effects on PTE mobility in soils were tested after the addition of organic amendments (Grüter et al. 2019; Luo et al. 2021; Mohseni et al. 2021; Sun et al. 2022; Zhao et al. 2022), biochar (Bidar et al. 2019; Babalola and Zhang 2021; Gao et al. 2022a, b), and inorganic amendments and sludge (Neu et al. 2018; Xu et al. 2019b; Manzano et al. 2019; Zhang et al. 2019; Mohseni et al. 2021; Senila et al. 2022; Zhang et al. 2022a; Zhang et al. 2022b). Other authors used DGT to assess the effect of soil moisture in PTE mobility (Li et al. 2015; Wang et al. 2020; Zhao et al. 2021; Wang et al. 2022b) or to evaluate the effect of hyperaccumulator plants in PTE mobilization in rhizosphere (Senila et al. 2013; Li et al. 2016; Zheng et al. 2019). Also, the effect of aging periods on PTE mobility in soil was evaluated by DGT (Ma et al. 2022).
Organic amendments, as determined by DGT and other chemical extraction of uptake by crops, generally decreased the PTE mobility in soils. Thus, long-term fertilization with organic matter was found to reduce the Cd transfer in wheat. However, the Zn transfer was not reduced (Grüter et al. 2019). Luo et al. (2021) reported that five of the soil amendments used in their research reduced the transfer of Cd to rice, and that the DGT estimated with precision the Cd bioavailable to rice. In another study, the Zn contaminated soil was treated with sorghum, poultry manure, and clover residues (Mohseni et al. 2021). Generally, due to an increasing dissolved organic carbon and a decreasing soil pH, the bioavailability increased in the amended soil. However, sorghum residues reduced the phytotoxicity risk of Zn. Sun et al. (2022) studied the stabilization of Zn in agricultural soil after the application of organic fertilizer and zeolite. The authors used the DGT, DTPA extraction, and accumulation in Chinese cabbage biomass to assess the reduction of Zn mobility in soil after the treatments. Zhao et al. (2022) employed the DGT to monitor the Cd mobility in soils interacted with controlled-release fertilizers coated with microplastics.
Biochar, a biomaterial produced during the pyrolysis of biomass, was also applied as soil amendment in which the DGT was employed to assess the PTE mobility changes. Thus, Babalola and Zhang (2021) used DGT to evaluate the available concentrations of Pb, Cu, and Cd in soils treated with biochar and Delonix regia pod biomass. Biochar modified with magnetite nanoparticles was reported to reduce Cd bioavailability to rice (Gao et al. 2022a, b). The DGT study indicates that biochar modified with magnetite nanoparticles may decrease the replenish capacity of soils to soil pore waters and thus limit the crop uptake. Bidar et al. (2019) studied the immobilization of Cd, Cu, Pb, and Zn in contaminated brownfield and agricultural soils treated with wood biochar and iron grit.
Other studies were focused on the effects of inorganic amendments and sludge application on soil in PTE mobility. DGT was compared with other two analytical approaches to measure the possible changes in the availability of Cu, Zn, and As in polluted soils, after a co-application of paper sludge alkaline waste and iron sulfate (Manzano et al. 2019). The DGT was found to be a good alternative to assess metal mobility, with less technical requirements. Using DGT to measure Cd, Pb, and Zn bioavailability in soil treated with sludge, Mohseni et al. (2021) estimated that the sewage sludge treatment rises the resupply of these elements to soil solution and the effective concentration of Cd, Pb, and Zn. Senila et al. (2022) examined the influence of natural zeolite amendment to contaminated soil on heavy metal (Cd, Cr, Cu, Pb, and Zn) availability over a 3-month period of incubation. A decrease of Cd and Pb mobility in the soil solid phase from the samples treated with zeolite was observed. The immobilization of metals (Cd, Pb, Zn) and As in agricultural soils contaminated with drinking water treatment residues was tested in pot experiments (Neu et al. 2018) and assessed by DGT. Xu et al. (2019b) studied the effect of phosphate application to the agricultural soil on the availability of Pb. Using DGT, in situ solution extraction, and EDTA extraction methods, a mobilization effect of the amendment application on Pb in soil was observed. Zhang et al. (2019) reported the use of a sequential extraction procedure coupled with the DGT technique to assess the influence of the ferrihydrite dissolution/transformation process on the availability of As in soils. In another research, As was extracted from polluted paddy soils with ferrihydrite-loaded sand columns (Zhang et al. 2022a). The process was investigated using mesocosm coupled with DGT for in situ visualization. Zhang et al. (2022b) used the DGT to assess the effects of soil remediation by nanoscale zero-valent iron on heavy metal (Cr, Cu, Zn, Pb) bioavailability in soil.
The content of water in soil along with the way it is managed also has a role in PTE mobility. In a study, DGT was used to examine the effects of the soil-drying processes on metal availability in contaminated soil. Soil moisture influences the metal availability, but it is dependent on the metal species and soil types (Li et al. 2015). Three water-management treatments, namely continuous flooding, intermittent flooding, and non-flooding, were conducted in pot experiments to assess their effects on the Cd phytoavailaility in three types of paddy soils and the Cd accumulation in rice (Wang et al. 2020). The DGT technique measured the available Cd in soil and provided the most trustworthy prediction for the Cd accumulation in rice. Zhao et al. (2021) studied the effect of sulfur on soil Cd mobility under flooded conditions in the soil-rice system with the aid of the DGT technique. It was found that the synergistic effect of Fe and S reduced the mobility of Cd. Wang et al. (2022b) used DGT and soil pore water sampling to explore the impacts of various types of S application on the bioavailability of Cd. It was reported that soluble and labile Cd concentration was immediately fixed in soil after flooding, but activated next to the rice transplantation.
Hyperaccumulator plants can modify the PTE mobility in rhizosphere by their roots. The effects of phytoextraction using the hyperaccumulator S. plumbizincicola on Cd and Zn availability, desorption kinetics, and speciation in contaminated soils were examined by chemical extraction and by the DIFS model (Li et al. 2016). The effects of two fern species on the mobility changes in rhizosphere soil were investigated in pot experiments using DGT (Senila et al. 2013). An increase of As labile fraction was observed in the rhizosphere of the fern species, a hyperaccumulator, in the grown plant. Zheng et al. (2019) studied the influence of biochar addition on the phytoextraction process of an As hyperaccumulator. A pot experiment was conducted to investigate the biochar effect on the As transfer in Pteris vittata fern. The DGT technique was utilized to characterize the migration of As in soil. The results showed that phytoextraction meaningly decreases Cd and Zn availability and that the hyperaccumulator has a significant role in the mobilization of less available metal fractions by repeated phytoextraction.
The effect of different aging times on Cd bioavailability in soil was evaluated by means of chemical extractions, DGT technique, and biological indicators (toxicity to barley) (Ma et al. 2022). It was observed that aging decreases the Cd bioavailability and transfer to barley. The plant root is more appropriate for predicting the Cd transfer from soil, while the plant shoot can well assess the toxic effect of Cd stress on plants. A better evaluation of the Cd bioavailability to barley was reported for the DGT, compared to the classical chemical extractions.
Studies using DGT as tool for the evaluation of Hg bioavailability in soil
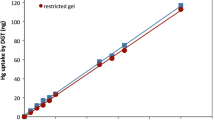
Hg exhibits several features which differentiate its assessment in soil from other metals. Five recent studies on Hg in soil measured by DGT were examined in this review. In the case of Hg, the specific resin gels used in the DGT units contain thiol groups with a high affinity for Hg, such as spheron-thiol or 3-mercaptopropyl-functionalized silica gel (Turull et al. 2019a). In order to differentiate the inorganic and organic labile Hg species in soil, Turull et al. (2019a) used open and restricted diffusive gels. Polyacrylamide crosslinked with agarose was used as open pore diffusive gel, while, as restricted pore layer, a polyacrylamide crosslinked with bis-acrylamide gel was prepared. Both open and restricted gels provided linear relationships between the mass of Hg collected in the resin gels and the Hg concentration in the solution. The two types of DGT units were successfully used to measure inorganic and organic Hg species in soil and were confirmed to effectively predict the Hg uptake by lettuce plants. The DGT technique was also used to measure the bioavailability of Hg in agricultural soils amended with organic fertilizers (biochar and compost), and predicted its uptake by lettuce (Turull et al. 2019b). Both open and restricted diffusive layers were also used in this study to test organic and inorganic Hg species in soils.
The DGT and the DIFS instruments were implemented to examine the Hg resupply kinetics, diffusion, and availability in a paddy soil exposed to flood-drain-reflood management and straw application (Yang et al. 2023). The straw amendment restricted the bioavailability of Hg in the porewater by lessening its resupply capacity, while the transformation into MeHg was substantially enhanced after straw application. Pelcová et al. (2019) studied the mercury bioavailability to Pisum sativum L. in soils and compared the bioaccumulation with the DGT measurements. Significantly positive correlations were reported between the Hg-DGT flux and the Hg flux into the plant root, leaf, and stem. The DGT total and labile Hg concentrations in garden soils from a former nonferrous metal mining area and the transfer to crops were evaluated (Senila et al. 2023). It was found that, on average, 84% of the Hg content in soil solution was found in its DGT-labile form.
Conclusions and perspectives
The DGT technique relates the diffusive flux of analyte to the gradient of the concentration. The current review provides an update of the existing DGT applications in the agricultural field, mainly for assessing the nutrients’ and PTEs’ labile fractions and bioavailability in soil. Recent DGT research focus not only on the nutrients’ bioavailability in agricultural soils, mostly P, Se, K, Mn, and nitrogen compounds, but also on the soil and plant contamination with potentially toxic elements, such as Hg, Cd, Pb, Zn, Cu, Cr, and As. Most of the reviewed literature reported significant positive correlations of the DGT labile fraction with the concentration in soil solution and with the bioaccumulated concentrations of the studied analytes in various biological indicators. In order to obtain accurate results, specific binding and diffusive layers were developed for certain analytes, such as Hg, Cd, Zn, P, and Se, although, in most cases, Chelex-based binding gels for cations and ferrihydrite-based hydrogels for oxyanions were mainly used. Generally, the DGT technique was more predictive for the soil mobility and bioaccumulation rates of the analytes than the classical extraction and fractionation methods, while being more compliant to the safety and environmental requirements.
Currently, studies on DGT deployments in situ are limited mainly to flooded soils. However, the development of DGT for in situ deployment for non-flooded soils is particularly interesting. Clear procedures for controlling soil parameters during the DGT deployments should be established for this. Another possible future development is the production of tools capable of expanding the number of target analytes that can be simultaneously determined.
Because DGT has several limitations in mimicking some key processes in dynamic environments, future research to link DGT results with bioassays and equilibrium-based methods will offer a more complete understanding of element bioavailability. Although DGT is a very promising technique and has several unique features which offers information on element bioavailability and toxicity that can be integrated to improve the regulatory work in toxicological and environmental fields, extensive research to produce larger datasets is still necessary (Guan 2019).
DGT as a passive sampling tool is already well integrated in the general tendency in analytical chemistry to achieve greener methodologies. It can improve the limits of quantification, eliminate the interferences by separation of analytes from the complex matrices, thus contributing to the improvement of the performances of analytical methods, reduces the number of necessary steps of analytical procedures, can replace or eliminate the use of toxic reagents in sample preparation, and can be used for performing multi-parameter analysis. Since different binding gels must still be used for the analysis of different nutrients/PTEs, the production of gels capable of expanding the number of target analytes simultaneously determined can be a future development.
Data availability
The relevant data from this research are available in the authors’ repositories.
References
Almendros P, Gonzalez D, Ibanez MA, Fernandez MD, Garcia-Gomez C, Smolders E, Obrador A (2020) Can diffusive gradients in thin films (DGT) technique and chemical extraction methods successfully predict both Zn bioaccumulation patterns in plant and leaching to groundwater in soils amended with engineered ZnO nanoparticles? J Soil Sci Plant Nutr 20:1714–1731. https://doi.org/10.1007/s42729-020-00241-x
Almendros P, Gonzalez D, Ibanez MA, Smolders E, Fernandez MD, Garcia-Gomez C, Obrador A (2022) Influence of ZnO particle size and soil characteristics on the estimation of long-term Zn bioavailability by chemical extraction methods and diffusive gradients in thin-films (DGT). J Soil Sci Plant Nutr 22:3901–3913. https://doi.org/10.1007/s42729-022-00938-1
Babalola B, Zhang H (2021) Diffusive gradient in thin film technique as tool for assessment of metal availability and kinetics of resupply in remediated soils. Groundw Sustain Dev 12:100493. https://doi.org/10.1016/j.gsd.2020.100493
Bai X, Ye W, Zhou Y, Ruan X, Wang J, Li W, Zhang P (2023) Comparison between diffusive gradients in thin film technology (DGT) and traditional methods for prediction of plant available heavy metals in agricultural soil. J Soils Sediments 23:1501–1510. https://doi.org/10.1007/s11368-022-03410-w
Bidar G, Pelfrêne A, Louvel B, Janus A, Douay F (2019) Influence of amendments on metal environmental and toxicological availability in highly contaminated brownfield and agricultural soils. Environ Sci Pollut Res Int 26:33086–33108. https://doi.org/10.1007/s11356-019-06295-4
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691. https://doi.org/10.1016/j.heliyon.2020.e04691
Chen H, Yuan J, Chen G, Zhao X, Wang S, Wang D, Wang L, Wang Y, Wang Y (2022) Long-term biochar addition significantly decreases rice rhizosphere available phosphorus and its release risk to the environment. Biochar 4:54. https://doi.org/10.1007/s42773-022-00178-7
Chen R, Gao T, Cheng N, Ding G, Wang Q, Shi R, Hu G, Cai X (2021) Application of DGT/DIFS to assess bioavailable Cd to maize and its release in agricultural soils. J Hazard Mater 411:124837. https://doi.org/10.1016/j.jhazmat.2020.124837
Chen Z, Imran M, Jing G, Wang W, Huang B, Li Y, Zhang Y, Yang Y, Lu Q, Zhang Z, Antoniadis V, Shaheen SM, Bolan N, Rinklebe J (2023) Toxic elements pollution risk as affected by various input sources in soils of greenhouses, kiwifruit orchards, cereal fields, and forest/grassland. Environ Pollut 338:122639. https://doi.org/10.1016/j.envpol.2023.122639
Dai Y, Nasir M, Zhang Y, Wu H, Guo H, Lv J (2017) Comparison of DGT with traditional methods for assessing cadmium bioavailability to Brassica chinensis in different soils. Sci Rep 7:14206. https://doi.org/10.1038/s41598-017-13820-3
Davison W (2016) Diffusive gradients in thin-films for environmental measurements. Cambridge University Press, Cambridge
Davison W, Hooda PS, Zhang H, Edwards AC (1999) DGT measured fluxes as surrogates for uptake of metals by plants. Adv Environ Res 3(4):550–555
Davison W, Zhang H (1994) In situ speciation measurements of trace components in natural waters using thin-film gels. Nature 367:546–548. https://doi.org/10.1038/367546a0
Davison W, Zhang H (2012) Progress in understanding the use of difusive gradients in thin flms (DGT) back to basics. Environ Chem 9:1–13. https://doi.org/10.1071/EN11084
Davison W, Zhang H (2016) Diffusion layer properties. In: William D (ed) Diffusive gradients in thin-films for environmental measurements. Cambridge University Press, Cambridge, pp 32–65
Degryse F, Smolders E (2016) DGT and bioavailability. In: Davison W (ed) Diffusive gradients in thin-films for environmental measurements. Cambridge University Press, Cambridge, pp 216–262
Degryse F, Smolders E, Zhang H, Davison W (2009) Predicting availability of mineral elements to plants with the DGT technique: a review of experimental data and interpretation by modelling. Environ Chem 6:198–218 http://150.229.72.10/paper/EN09010.html
Dinh QT, Zhou F, Wang M, Peng Q, Wang M, Qi M, Tran TAT, Chen H, Liang D (2021) Assessing the potential availability of selenium in the soil-plant system with manure application using diffusive gradients in thin-films technique (DGT) and DOM-Se fractions extracted by selective extractions. Sci Total Environ 763:143047. https://doi.org/10.1016/j.scitotenv.2020.143047
Dippong T, Hoaghia MA, Senila M (2022) Appraisal of heavy metal pollution in alluvial aquifers. Study case on the protected area of Ronișoara Forest, Romania. Ecol Indic 143:109347. https://doi.org/10.1016/j.ecolind.2022.109347
Galhardi JA, de Mello JWV, Wilkinson KJ (2020) Bioaccumulation of potentially toxic elements from the soils surrounding a legacy uranium mine in Brazil. Chemosphere 261:127679. https://doi.org/10.1016/j.chemosphere.2020.127679
Gao B, Chen Q, Liu K, Li F, Fang L, Zhu Z, Tran AT, Peng J (2022a) Biogeochemical Fe(II) generators as a new strategy for limiting Cd uptake by rice and its implication for agricultural sustainability. Sci Total Environ 820:153306. https://doi.org/10.1016/j.scitotenv.2022.153306
Gao B, Gao L, Xu D (2022b) New insight for the diffusion–resupply kinetics of Cr(VI) in contaminated soil using DGT/DIFS. Ecotox Environ Safe 242:113946. https://doi.org/10.1016/j.ecoenv.2022.113946
Gao Y, Zhou C, Gaulier C, Bratkic A, Galceran J, Puy J, Zhang H, Leermakers M, Baeyens W (2019) Labile trace metal concentration measurements in marine environments: from coastal to open ocean areas. TrAC Trends Anal Chem 116:92–101. https://doi.org/10.1016/j.trac.2019.04.027
Gramlich A, Tandy S, Gauggel C, Lopez M, Perla D, Gonzalez V, Schulin R (2018) Soil cadmium uptake by cocoa in Honduras. Sci Total Environ 612:370–378. https://doi.org/10.1016/j.scitotenv.2017.08.145
Grüter R, Costerousse B, Mayer J, Mäder P, Thonar C, Frossard E, Schulin R, Tandy S (2019) Long-term organic matter application reduces cadmium but not zinc concentrations in wheat. Sci Total Environ 669:608–620. https://doi.org/10.1016/j.scitotenv.2019.03.112
Guan D-X (2019) Diffusive Gradients in Thin-Films (DGT): An effective and simple tool for assessing contaminant bioavailability in waters, soils and sediments. In: Nriagu J (ed) Encyclopedia of Environmental Health (Second Edition). Elsevier, pp 111–124. https://doi.org/10.1016/B978-0-12-409548-9.11403-4
Guan DX, He SX, Li G, Teng HH, Ma LQ (2022) Application of diffusive gradients in thin-films technique for speciation, bioavailability, modeling and mapping of nutrients and contaminants in soils. Crit Rev Environ Sci Technol 52(17):3035–3079. https://doi.org/10.1080/10643389.2021.1900765
Guan DX, Zheng JL, Luo J, Zhang H, Davison W, Ma LQ (2017) A diffusive gradients in thin-films technique for the assessment of bisphenols desorption from soils. J Hazard Mater 331:321–328. https://doi.org/10.1016/j.jhazmat.2017.02.053
Guo J, Wei Z, Zhang C, Li C, Dai L, Lu X, Xiao K, Mao X, Yang X, Jing Y, Zhang J, Chen W, Qi S (2023) Characteristics and DGT based bioavailability of cadmium in the soil–crop systems from the east edge of the Dongting Lake, China. Int J Environ Res Public Health 20:30. https://doi.org/10.3390/ijerph20010030
Harper MP, Davison W, Tych W (2000) DIFS– A modelling and simulation tool for DGT induced trace metal remobilisation in sediments and soils. Environ Model Softw 15:55–66. https://doi.org/10.1016/S1364-8152(99)00027-4
Hill B, Santner J, Spiegel H, Puschenreiter M (2021) Wenzel WW (2001) Diffusive gradients in thin films predicts crop response better than calcium-acetate-lactate extraction. Nutr Cycl Agroecosyst 121:227–240. https://doi.org/10.1007/s10705-021-10173-2
Jarosch KA, Santner J, Parvage MM, Gerzabek MH, Zehetner F, Kirchmann H (2018) Four soil phosphorus (P) tests evaluated by plant P uptake and P balancing in the Ultuna long-term field experiment. Plant Soil Environ 9:441–447. https://doi.org/10.17221/313/2018-PSE
Jiang T, Yu T, Qi H, Li F, Yang Z (2022) Analysis of phosphorus and sulfur effect on soil selenium bioavailability based on diffusive gradients in thin films technique and sequential extraction. Chemosphere 302:134831. https://doi.org/10.1016/j.chemosphere.2022.134831
Jiang Y, Hu B, Shi H, Yi L, Chen S, Zhou Y, Cheng J, Huang M, Yu W, Shi Z (2023) Pollution and risk assessment of potentially toxic elements in soils from industrial and mining sites across China. J Environ Manag 336:117672. https://doi.org/10.1016/j.jenvman.2023.117672
Jolley DF, Mason S, Gao Y, Zhang H (2016) Practicalities of working with DGT. In: William D, Zhang H (eds) Diffusive gradients in thin-films for environmental measurements. Cambridge University Press, Cambridge
Kalkhajeh YK, Sorensen H, Huang B, Guan D-X, Luo J, Hu W, Holm PE, Hansen HCB (2018) DGT technique to assess P mobilization from greenhouse vegetable soils in China: a novel approach. Sci Total Environ 630(331):339. https://doi.org/10.1016/j.scitotenv.2018.02.228
Kang L, Zhang G, Chu G (2021) Split delivering phosphorus via fertigation to a calcareous soil increased P availability and maize yield (Zea mays L.) by reducing P fixation. J Soils Sediments 21:2287–2300. https://doi.org/10.1007/s11368-021-02914-1
Kodithuwakku K, Huang J, Doolette CL, Mason S, Boland J, Lombi E, Lehto NJ, Teasdale PR (2023) Evaluation of the diffusive gradients in thin-films (DGT) technique for measuring nitrate and ammonium in soil. Environ Chem 19:483–494. https://doi.org/10.1071/EN22107
Kuziemska B, Wysokinski A, Klej P (2023) The content, uptake and bioaccumulation factor of copper and nickel in grass depending on zinc application and organic fertilization. Agriculture 13:1676. https://doi.org/10.3390/agriculture13091676
Letho NJ (2016) Principles and application in soils and sediments. In: William D (ed) Diffusive gradients in thin-films for environmental measurements. Cambridge University Press, Cambridge, pp 146–173
Li D, Li W, Lu Q, Li Y, Li N, Xu H, Ren Z, Zhang Y, Wang J (2018) Cadmium bioavailability well assessed by DGT and factors influencing cadmium accumulation in rice grains from paddy soils of three parent materials. J Soils Sediments 18:2552–2561. https://doi.org/10.1007/s11368-018-1950-2
Li Y, Ajmone-Marsan F, Padoan E (2023) Combining DGT with bioaccessibility methods as tool to estimate potential bioavailability and release of PTEs in the urban soil environment. Sci Total Environ 857:159597. https://doi.org/10.1016/j.scitotenv.2022.159597
Li Z, Jia M, Wu L, Christie P, Luo Y (2016) Changes in metal availability, desorption kinetics and speciation in contaminated soils during repeated phytoextraction with the Zn/Cd hyperaccumulator Sedum plumbizincicola. Environ Pollut 209:123–131. https://doi.org/10.1016/j.envpol.2015.11.015
Li Z, Wu L, Zhang H, Luo Y, Christie P (2015) Effects of soil drying and wetting-drying cycles on the availability of heavy metals and their relationship to dissolved organic matter. J Soils Sediments 15:1510–1519. https://doi.org/10.1007/s11368-015-1090-x
Luo H, Du P, Shi J, Yang B, Liang T, Wang P, Chen J, Zhang Y, He Y, Jia X, Duan G, Li F (2021) DGT methodology is more sensitive than conventional extraction strategies in assessing amendment-induced soil cadmium availability to rice. Sci Total Environ 760:143949. https://doi.org/10.1016/j.scitotenv.2020.143949
Lyu C, Qin Y, Zhao Z, Liu X (2021) Characteristics of selenium enrichment and assessment of selenium bioavailability using the diffusive gradients in thin-films technique in seleniferous soils in Enshi, Central China. Environ Pollut 273:116507. https://doi.org/10.1016/j.envpol.2021.116507
Ma P, Tian T, Dai Z, Shao T, Zhang W, Liu M (2022) Assessment of Cd bioavailability using chemical extraction methods, DGT, and biological indicators in soils with different aging times. Chemosphere 296:133931. https://doi.org/10.1016/j.chemosphere.2022.133931
Manzano R, Rosende M, Leza A, Esteban E, Peñalosa JM, Miró M, Moreno-Jiménez E (2019) Complementary assessment of As, Cu and Zn environmental availability in a stabilised contaminated soil using large-bore column leaching, automatic microcolumn extraction and DGT analysis. Sci Total Environ 690:217–225. https://doi.org/10.1016/j.scitotenv.2019.06.523
Marrugo-Madrid S, Turull M, Zhang H, Díez S (2021) Diffusive gradients in thin films for the measurement of labile metal species in water and soils: a review. Environ Chem Lett 19:3761–3788. https://doi.org/10.1007/s10311-021-01246-3
Mohseni A, Heidari S, Raei B, Moftakharzadeh SA, Bidast S (2022) Determination of poultry manure and plant residues effects on Zn bioavailable fraction in contaminated soil via DGT technique. Arch Environ Contam Toxicol 82(1):72–81. https://doi.org/10.1007/s00244-021-00901-8
Mohseni A, Reyhanitabar A, Heidari S (2021) Efficiency of a new allylagarose-assembled DGT in measuring Cd, Zn, and Pb bioavailability in sludge-treated soil. Environ Technol Innov 24:102034. https://doi.org/10.1016/j.eti.2021.102034
Mundus S, Carstensen A, Husted S (2017) Predicting phosphorus availability to spring barley (Hordeum vulgare) in agricultural soils of Scandinavia. Field Crop Res 212:1–10. https://doi.org/10.1016/j.fcr.2017.06.026
National Research Council (2003) Bioavailability of contaminants in soils and sediments: processes, tools, and applications. The National Academies Press, Washington, DC. https://doi.org/10.17226/10523ISO
Nawara S, van Dael T, De Cooman E, Elsen A, Merckx R, Smolders E (2018) Testing soil phosphorus in a depleting P scenario: an accelerated soil mining experiment. Eur J Soil Sci 69:804–815. https://doi.org/10.1111/ejss.12684
Nawara S, van Dael T, Merckx R, Amery F, Elsen A, Odeurs W, Vandendriessche H, Mcgrath S, Roisin C, Jouany C, Pellerin S, Denoroy P, Eichler-Lobermann B, Borjesson G, Goos P, Akkermans W, Smolders E (2017) A comparison of soil tests for available phosphorus in long-term field experiments in Europe. Eur J Soil Sci 68:873–885. https://doi.org/10.1111/ejss.12486
Neu S, Müller I, Brackhage C, Gałązka R, Siebielec G, Puschenreiter M, Dudel EG (2018) Trace elements bioavailability to Triticum aestivum and Dendrobaena veneta in a multielement-contaminated agricultural soil amended with drinking water treatment residues. J Soils Sediments 18:2259–2270. https://doi.org/10.1007/s11368-017-1741-1
Ngo LK, Pinch BM, Bennett WW, Teasdale PR, Jolley DF (2016) Assessing the uptake of arsenic and antimony from contaminated soil by radish (Raphanus sativus) using DGT and selective extractions. Environ Pollut 216:104–114. https://doi.org/10.1016/j.envpol.2016.05.027
Ngo LK, Price HL, Bennett WW, Teasdale PR, Jolley DF (2020) DGT and selective extractions reveal differences in arsenic and antimony uptake by the white icicle radish (Raphanus sativus). Environ Pollut 259:113815. https://doi.org/10.1016/j.envpol.2019.113815
Ning J, Arai Y, Shen J, Wang R, Ai S (2021) Effects of phosphorus on nitrification process in a fertile soil amended with urea. Agriculture 11:523. https://doi.org/10.3390/agriculture11060523
Nobile CM, Bravin MN, Tillard E, Becquer T, Paillat J-M (2018) Phosphorus sorption capacity and availability along a toposequence of agricultural soils: effects of soil type and a decade of fertilizer applications. Soil Use Manag 34:461–471. https://doi.org/10.1111/sum.12457
Park SH, An J, Koutsospyros A, Moon DH (2023) Assessment of the stabilization of Cu-, Pb-, and Zn-contaminated fine soil using cockle shells, scallop shells, and starfish. Agriculture 13:1414. https://doi.org/10.3390/agriculture13071414
Pelcová P, Zouharová I, Ridošková A, Smolíková V (2019) Evaluation of mercury availability to pea parts (Pisum sativum L.) in urban soils: comparison between diffusive gradients in thin films technique and plant model. Chemosphere 234:373–378. https://doi.org/10.1016/j.chemosphere.2019.06.076
Peng Q, Li J, Wang D, Wei TJ, Chen CL, Liang D-L (2019) Effects of ageing on bioavailability of selenium in soils assessed by diffusive gradients in thin-films and sequential extraction. Plant Soil 436:159–171. https://doi.org/10.1007/s11104-018-03920-y
Peng Q, Wang D, Wang M, Zhou F, Yang W, Liu Y, Liang D (2020) Prediction of selenium uptake by pak choi in several agricultural soils based on diffusive gradients in thin-films technique and single extraction. Environ Pollut 256:113414. https://doi.org/10.1016/j.envpol.2019.113414
Peng Q, Wang M, Cui Z, Huang J, Chen C, Guo L, Liang D (2017) Assessment of bioavailability of selenium in different plant-soil systems by diffusive gradients in thin-films (DGT). Environ Pollut 225:637–643. https://doi.org/10.1016/j.envpol.2017.03.036
Petrean IA, Micle V, Sur IM, Șenilă M (2023) Characterization of sterile mining dumps by the ICP-OES analytical method: a case study from Baia Mare mining area (Maramures, Romania). Sustainability 15:1158. https://doi.org/10.3390/su15021158
Rashid A, Schutte BJ, Ulery A, Deyholos MK, Sanogo S, Lehnhoff EA, Beck L (2023) Heavy metal contamination in agricultural soil: environmental pollutants affecting crop health. Agronomy 13:1521. https://doi.org/10.3390/agronomy13061521
Ren S, Wang Y, Sun D, Bekele TG, Dong F, Zhao H, Tan F (2022) Simultaneous evaluation of kinetic release of labile arsenic and phosphorus in agricultural soils using cerium oxide-based DGT. Sci Total Environ 807:151039. https://doi.org/10.1016/j.scitotenv.2021.151039
Roba C, Roşu C, Piştea I, Ozunu A, Baciu C (2016) Heavy metal content in vegetables and fruits cultivated in Baia Mare mining area (Romania) and health risk assessment. Environ Sci Pollut Res 23:6062–6073. https://doi.org/10.1007/s11356-015-4799-6
Santner J, Larsen M, Kreuzeder A, Glud RN (2015) Two decades of chemical imaging of solutes in sediments and soils – a review. Anal Chim Acta 878:9–42. https://doi.org/10.1016/j.aca.2015.02.006
Senila M (2014) Real and simulated bioavailability of lead in contaminated and uncontaminated soils. J Environ Health Sci Eng 12:108. https://doi.org/10.1186/2052-336X-12-108
Senila M, Cadar O, Senila L, Angyus BS (2022) Simulated bioavailability of heavy metals (Cd, Cr, Cu, Pb, Zn) in contaminated soil amended with natural zeolite using diffusive gradients in thin-films (DGT) technique. Agriculture 12:321. https://doi.org/10.3390/agriculture12030321
Senila M, Levei EA, Frentiu T, Mihali C, Angyus SB (2023) Assessment of mercury bioavailability in garden soils around a former nonferrous metal mining area using DGT, accumulation in vegetables, and implications for health risk. Environ Monit Assess 195:1554. https://doi.org/10.1007/s10661-023-12144-2
Senila M, Resz MA, Senila L, Torok I (2024) Application of diffusive gradients in thin-films (DGT) for assessing the heavy metals mobility in soil and prediction of their transfer to Russula virescens. Sci Total Environ 909:168591. https://doi.org/10.1016/j.scitotenv.2023.168591
Senila M, Tanaselia C, Rimba E (2013) Investigatins on arsenic mobility changes in rizosphere of two ferns species using DGT technique. Carpathian J Earth Environ Sci 8:145–154
Smolders E, Wagner S, Prohaska T, Irrgeher J, Santner J (2020) Sub-millimeter distribution of labile trace element fluxes in the rhizosphere explains differential effects of soil liming on cadmium and zinc uptake in maize. Sci Total Environ 738:140311. https://doi.org/10.1016/j.scitotenv.2020.140311
Sochaczewski Ł, Tych W, Davison B, Zhang H (2007) 2D DGT induced fluxes in sediments and soils (2D DIFS). Environ Model Softw 22:14–23. https://doi.org/10.1016/j.envsoft.2005.09.008
Sun L, Li S, Gong P, Song K, Zhang H, Sun Y, Qin Q, Zhou B, Xue Y (2022) Stabilization of zinc in agricultural soil originated from commercial organic fertilizer by natural zeolite. Int J Environ Res Public Health 19:1210. https://doi.org/10.3390/ijerph19031210
Tandy S, Mundus A, Yngvesson J, de Bang TC, Lombi E, Schjoerring JK, Husted S (2011) The use of DGT for prediction of plant available copper, zinc and phosphorus in agricultural soils. Plant Soil 346:167–180. https://doi.org/10.1007/s11104-011-0806-y
Thalassinos G, Petropoulos SA, Grammenou A, Antoniadis V (2023) Potentially toxic elements: a review on their soil behavior and plant attenuation mechanisms against their toxicity. Agriculture 13:1684. https://doi.org/10.3390/agriculture13091684
Tian K, Xing Z, Guoming L, Wang H, Jia M, Hu W, Huang B (2018) Cadmium phytoavailability under greenhouse vegetable production system measured by diffusive gradients in thin films (DGT) and its implications for the soil threshold. Environ Pollut 241:412–421. https://doi.org/10.1016/j.envpol.2018.05.086
Tuntrachanida J, Wisawapipat W, Aramrak S, Chittamart N, Klysubun W, Amonpattaratkit P, Duboc O, Wenzel WW (2022) Combining spectroscopic and flux measurement techniques to determine solid-phase speciation and solubility of phosphorus in agricultural soils. Geoderma 410:115677. https://doi.org/10.1016/j.geoderma.2021.115677
Turull M, Fontàs C, Díez S (2019a) Diffusive gradient in thin films with open and restricted gels for predicting mercury uptake by plants. Environ Chem Lett 17:1353–1358. https://doi.org/10.1007/s10311-019-00864-2
Turull M, Fontàs C, Díez S (2019b) Conventional and novel techniques for the determination of Hg uptake by lettuce in amended agricultural peri-urban soils. Sci Total Environ 668:40–46. https://doi.org/10.1016/j.scitotenv.2019.02.244
Turull M, Fontàs C, Díez S (2021) Effect of different amendments on trace metal bioavailability in agricultural soils and metal uptake on lettuce evaluated by diffusive gradients in thin films. Environ Technol Innov 21:101319. https://doi.org/10.1016/j.eti.2020.101319
Vogel C, Doolette A, Huang J (2021) Combining diffusive gradients in thin-films (DGT) and 31P NMR spectroscopy to determine phosphorus species in soil. Agric Environ Lett e20068. https://doi.org/10.1002/ael2.20068
Vogel C, Sekine R, Steckenmesser D, Lombi E, Steffens D, Adam C (2017) Phosphorus availability of sewage sludge-based fertilizers determined by the diffusive gradients in thin films (DGT) technique. J Plant Nutr Soil Sci 180:594–601. https://doi.org/10.1002/jpln.201600531
Wang J, Li D, Lu Q, Zhang Y, Xu H, Wang X, Yongtao L (2020) Effect of water-driven changes in rice rhizosphere on Cd lability in three soils with different pH. J Environ Sci 87:82–92. https://doi.org/10.1016/j.jes.2019.05.020
Wang M, Cui Z, Xue M, Peng Q, Zhou F, Wang D, Dinh QT, Liu Y, Liang D (2019a) Assessing the uptake of selenium from naturally enriched soils by maize (Zea mays L.) using diffusive gradients in thin-films technique (DGT) and traditional extractions. Sci Total Environ 689:1–9. https://doi.org/10.1016/j.scitotenv.2019.06.346
Wang Y, Chen H, Wang L, Zhu W, Yuan J, Jaisi DP, Zhao X, Wang S (2021) Using diffusive gradients in thin films technique for in-situ measurement of labile phosphorus around Oryza sativa L. roots in flooded paddy soils. Pedosphere 31:76–82. https://doi.org/10.1016/S1002-0160(20)60009-1
Wang Y, Yuan J-H, Chen H, Zhao X, Wang D, Wang S-Q, Ding S-M (2019b) Small-scale interaction of iron and phosphorus in flooded soils with rice growth. Sci Total Environ 669:911–919. https://doi.org/10.1016/j.scitotenv.2019.03.054
Wang Z, Er Q, Zhang C, Liu J, Liang X, Zhao Y (2023b) A new DGT technique based on nano-sized Mg2Al layered double hydroxides with DTPA for sampling of eight anionic and cationic metals. Environ Sci Pollut Res 30:37679–37690. https://doi.org/10.1007/s11356-022-24905-6
Wang Z, Liu W, Liu J, Liu X, Liu R, Zhao Y (2022b) Differences and mechanism of dynamic changes of Cd activity regulated by polymorphous sulfur in paddy soil. Chemosphere 291:133055. https://doi.org/10.1016/j.chemosphere.2021.1tur33055
Wang Z, Liu W, Zhang C, Liu X, Liang X, Liu R, Zhao Y (2023a) Mechanisms of S cooperating with Fe and Mn to regulate the conversion of Cd and Cu during soil redox process revealed by LDHs-DGT technology. Sci Total Environ 867:161431. https://doi.org/10.1016/j.scitotenv.2023.161431
Wang Z, Liu X, Liang X, Dai L, Li Z, Liu R, Zhao Y (2022a) Flooding-drainage regulate the availability and mobility process of Fe, Mn, Cd, and As at paddy soil. Sci Total Environ 817:152898. https://doi.org/10.1016/j.scitotenv.2021.152898
Wei T-J, Guan D-X, Li X-Y, Hao Y-L, Teng HH, Yang J-F, Xu Y-Y, Li G (2022) Analysis of studies on environmental measurements using diffusive gradients in thin-films (DGT) from 1994 to 2020. J Soils Sediments 22:1069–1079. https://doi.org/10.1007/s11368-022-03168-1
Wen Y, Li W, Yang Z, Zhuo X, Guan D-X, Song Y, Guo C, Ji J (2020) Evaluation of various approaches to predict cadmium bioavailability to rice grown in soils with high geochemical background in the karst region, Southwestern China. Environ Pollut 258:113645. https://doi.org/10.1016/j.envpol.2019.113645
Wenzel WW, Mesmer C, Florida EJ, Puschenreiter M, Kirchmann H (2022) Wheat yield prediction by zero sink and equilibrium-type soil phosphorus tests. Pedosphere 32:543–554. https://doi.org/10.1016/S1002-0160(21)60049-8
Wu L, Zhou J, Zhou T, Li Z, Jiang J, Zhu D, Hou J, Wang Z, Luo Y, Christie P (2018) Estimating cadmium availability to the hyperaccumulator Sedum plumbizincicola in a wide range of soil types using a piecewise function. Sci Total Environ 637-638:1342–1350. https://doi.org/10.1016/j.scitotenv.2018.04.386
Wu Y, Wang Z, Xu L, Feng W, Fan H (2021) Temporal responses of hydrochemical variables and dissolved Fe(II) to flooding at a lake riparian wetland under different vegetation revealing by high resolution DGT. J Environ Manag 294:112930. https://doi.org/10.1016/j.jenvman.2021.112930
Xiao W, Ye X, Zhu Z, Zhang Q, Zhao S, Chen D, Fang X, Gao N, Hu J (2020) Evaluation of cadmium (Cd) transfer from paddy soil to rice (Oryza sativa L.) using DGT in comparison with conventional chemical methods: derivation of models to predict Cd accumulation in rice grains. Environ Sci Pollut Res 27:14953–14962. https://doi.org/10.1007/s11356-020-07976-1
Xu D, Gao B, Chen S, Peng W, Zhang M, Qu X, Gao L, Li Y (2019a) Release risk assessment of trace metals in urban soils using in-situ DGT and DIFS model. Sci Total Environ 694:133624. https://doi.org/10.1016/j.scitotenv.2019.133624
Xu Q, Ye B, Mou X, Ye J, Liu W, Luo Y, Shi J (2019b) Lead was mobilized in acid silty clay loam paddy soil with potassium dihydrogen phosphate (KDP) amendment. Environ Pollut 255:113179. https://doi.org/10.1016/j.envpol.2019.113179
Yang C, Lu S (2022) The dynamic changes of phosphorus availability in straw/ biochar-amended soils during the rice growth revealed. J Soils Sediments 22:957–967. https://doi.org/10.1007/s11368-021-03131-6
Yang X, Li Z, Wang T, Yang Z, Wen X, Yang K, Huang Y, Chen W, He Y, Shi X, Zhang C, Yu Z (2023) Resupply, diffusion, and bioavailability of Hg in paddy soil-water environment with flood-drain-reflood and straw amendment. Environ Res 231:116127. https://doi.org/10.1016/j.envres.2023.116127
Yi Z, Lehto N, Robinson BH, Cavanagh J-A (2020) Environmental and edaphic factors affecting soil cadmium uptake by spinach, potatoes, onion and wheat. Sci Total Environ 713:136694. https://doi.org/10.1016/j.scitotenv.2020.136694
Zhang H, Davison W (1995) Performance characteristics of difusion gradients in thin flms for the in situ measurement of trace metals in aqueous solution. Anal Chem 67:3391–3400. https://doi.org/10.1021/ac00115a005
Zhang H, Davison W (2015) Use of difusive gradients in thin-flms for studies of chemical speciation and bioavailability. Environ Chem 12:85–101. https://doi.org/10.1071/EN14105
Zhang H, Davison W, Knight B, McGrath S (1998) In situ measurements of solution concentrations and fluxes of trace metals in soils using DGT. Environ Sci Technol 3:704–710. https://doi.org/10.1021/es9704388
Zhang H, Zhao FJ, Sun B, Davison W, McGrath SP (2001) A new method to measure effective soil solution concentration predicts copper availability to plants. Environ Sci Technol 35(12):2602–2607. https://doi.org/10.1021/es000268q
Zhang J, Wen J, Zhang T, Zhang Y, Peng Z, Tang C, Wang Y, Su S, Zhang N, Zeng X (2023) Effects of five–year inorganic and organic fertilization on soil phosphorus availability and phosphorus resupply for plant P uptake during maize growth. Agriculture 13:858. https://doi.org/10.3390/agriculture13040858
Zhang R, Huang B, Zeng H, Wang X, Peng B, Yu H, Guo W (2022a) Arsenic extraction from seriously contaminated paddy soils with ferrihydrite-loaded sand columns. Chemosphere 307:135744. https://doi.org/10.1016/j.chemosphere.2022.135744
Zhang T, Xia B, Lu Y, Zhang X, Chen H, Ying R, Jin S (2022b) Assessment of the effects of heavy metals in soils after removal by nanoscale zero-valent iron with three methods. Sustainability 14:2273. https://doi.org/10.3390/su14042273
Zhang T, Zeng X, Zhang H, Lin Q, Su S, Wang Y, Bai L, Wu C (2019) The effect of the ferrihydrite dissolution/transformation process on mobility of arsenic in soils: Investigated by coupling a two-step sequential extraction with the diffusive gradient in the thin films (DGT) technique. Geoderma 352:22–32. https://doi.org/10.1016/j.geoderma.2019.05.042
Zhang Y, Mason S, McNeill A, McLaughlin MJ (2013) Optimization of the diffusive gradients in thin films (DGT) method for simultaneous assay of potassium and plant-available phosphorus in soils. Talanta 113:123–129. https://doi.org/10.1016/j.talanta.2013.03.023
Zhang Y, Mason S, McNeill A, McLaughlin MJ (2014) Application of the diffusive gradients in thin films technique for available potassium measurement in agricultural soils: effects of competing cations on potassium uptake by the resin gel. Anal Chim Acta 42:27–34. https://doi.org/10.1016/j.aca.2014.07.023
Zhang Y, Nachimuthu G, Mason S, McLaughlin MJ, McNeill A, Bell MJ (2017) Comparison of soil analytical methods for estimating wheat potassium fertilizer requirements in response to contrasting plant K demand in the glasshouse. Sci Rep 7:11391. https://doi.org/10.1038/s41598-017-11681-4
Zhang Z, Shen F, Gu M, Liu Y, Pan L, Shohag MJI, Li T, Wei Y (2020) Evaluation of selenium bioavailability to Brassica juncea in representative Chinese soils based on diffusive gradients in thin-films (DGT) and chemical extraction methods. Int J Phytoremediation 1–11. https://doi.org/10.1080/15226514.2020.1774502
Zhao M, Li C, Zhang C, Han B, Wang X, Zhang J, Wang J, Cao B, Zhao Y, Chen Y, Zou G (2022) Typical microplastics in field and facility agriculture dynamically affect available cadmium in different soil types through physicochemical dynamics of carbon, iron and microbes. J Hazard Mater 440:129726. https://doi.org/10.1016/j.jhazmat.2022.129726
Zhao M, Liu X, Li Z, Liang X, Wang Z, Zhang C, Liu W, Liu R, Zhao Y (2021) Inhibition effect of sulfur on Cd activity in soil-rice system and its mechanism. J Hazard Mater 407:124647. https://doi.org/10.1016/j.jhazmat.2020.124647
Zheng C, Wang X, Liu J, Ji X, Huang B (2019) Biochar-assisted phytoextraction of arsenic in soil using Pteris vittata L. Environ Sci Pollut Res 26:36688–36697. https://doi.org/10.1007/s11356-019-06688-5
Zhou JW, Wu LH, Zhou T, Li Z, Sun XY, Luo YM, Christie P (2019) Comparing chemical extraction and a piecewise function with diffusive gradients in thin films for accurate estimation of soil zinc bioavailability to Sedum plumbizincicola. Eur J Soil Sci 70:1141–1152. https://doi.org/10.1111/ejss.12810
Zhou N, Chen Y, Wang J, Yang W, Wang Y (2023) Reducing chemical fertilizer application in greenhouse vegetable cultivation under different residual levels of nutrient. Agriculture 13:1174. https://doi.org/10.3390/agriculture13061174
Zulkernain NH, Uvarajan T, Ng CC (2023) Roles and significance of chelating agents for potentially toxic elements (PTEs) phytoremediation in soil: a review. J Environ Manag 341:117926. https://doi.org/10.1016/j.jenvman.2023.117926
Funding
This work was funded by a grant of the Romanian Ministry of Research and Innovation, CNCS/CCCDI-UEFISCDI, contract no. 733PED/2022, project number PN-III-P2-2.1-PED2021-0151, within PNCDI III.
Author information
Authors and Affiliations
Contributions
Marin Senila: funding acquisition, conceptualization, investigation, data curation, and writing—original draft, Eniko Kovacs: investigation, visualization, and writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study does not include any human or animal subjects.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Roberto Terzano
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Senila, M., Kovacs, E. Use of diffusive gradients in thin-film technique to predict the mobility and transfer of nutrients and toxic elements from agricultural soil to crops—an overview of recent studies. Environ Sci Pollut Res 31, 34817–34838 (2024). https://doi.org/10.1007/s11356-024-33602-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33602-5