Abstract
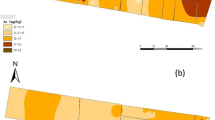
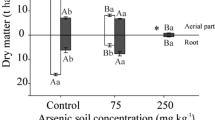
The alkaline nature of biochar provides a potential for soil arsenic (As) mobilization and, hence, enhancing efficiency of As phytoextraction by combining with As hyperaccumulator. To testify the feasibility and potential risk of the above strategy, biochar effect on As transfer in a paddy soil and accumulation in P. vittata was investigated in a pot experiment. By leaching soil (total As concentration 141.17 mg/kg) with simulated acid rain (pH 4.2), As the concentration in leaching eluate increased proportionally with increasing biochar ratio. Coincident with elevated soil As mobility, apparent enhancement in As uptake and translocation in P. vittata was determined with 1–5% biochar amendment after 40 days of plant growth. Furthermore, diffusive gradients in thin film (DGT) technique were employed to characterize any potential risk in vertical downward migration of As at 2-mm resolution. A significantly increasing profile of DGT-As ranging from on average 20 μg/L in CK to 50–100 μg/L in 1–3% biochar treatments was recorded over 0–60 mm depth, with 25–71% lower labile As in the rhizosphere than non-rhizosphere zone with few exceptions. As compared to Chinese quality standard for groundwater (Class IV 50 μg/L), biochar ratio at ≤ 1% was suggested for local water safety while actual application should take the physicochemical characteristic of tested soil into account. Our results demonstrated the biochar-assisted P. vittata phytoremediation can serve as an emerging pathway to enhance efficiency of soil As phytoextraction. The combination of DGT techniques and greenhouse assay provided a powerful tool for evaluating the gradient distribution of heavy metal in rhizosphere and accessing corresponding ecological risk at more precise scale.






Similar content being viewed by others
References
Ajayi AE, Holthusen D, Horn R (2016) Changes in microstructural behaviour and hydraulic functions of biochar amended soils. Soil Tillage Res 155:166–175
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals-concepts and applications. Chemosphere 91:869–881
Alloway BJ (2012) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Springer Science & Business Media, pp 101–104
Althobiti RA, Sadiq NW, Beauchemin D (2018) Realistic risk assessment of arsenic in rice. Food Chem 257:230–236
Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Slavkovich V, van Geen A, Graziano J, Ahsan H (2010) Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet 376:252–258
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Beesley L, Inneh OS, Norton GJ, Moreno-Jimenez E, Pardo T, Clemente R, Dawson JJC (2014) Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ Pollut 186:195–202
Brammer H, Ravenscroft P (2009) Arsenic in groundwater: a threat to sustainable agriculture in South and South-east Asia. Environ Int 35:647–654
Caille N, Zhao FJ, McGrath SP (2005) Comparison of root absorption, translocation and tolerance of arsenic in the hyperaccumulator Pteris vittata and the nonhyperaccumulator Pteris tremula. New Phytol 165:755–761
Cullen WR, Reimer KJ (1989) Arsenic speciation in the environment. Chem Rev 89:713–764
Ding S, Jia F, Xu D, Sun Q, Zhang L, Fan C, Zhang C (2011) High-resolution, two-dimensional measurement of dissolved reactive phosphorus in sediments using the diffusive gradients in thin films technique in combination with a routine procedure. Environ Sci Technol 45:9680–9686
Ding S, Han C, Wang Y, Yao L, Wang Y, Xu D, Sun Q, Williams PN, Zhang C (2015) In situ, high-resolution imaging of labile phosphorus in sediments of a large eutrophic lake. Water Res 74:100–109
Ding S, Wang Y, Zhang L, Xu L, Gong M, Zhang C (2016) New holder configurations for use in the diffusive gradients in thin films (DGT) technique. RSC Adv 6:88143–88156
Egene CE, Van Poucke R, Ok YS, Meers E, Tack FMG (2018) Impact of organic amendments (biochar, compost and peat) on Cd and Zn mobility and solubility in contaminated soil of the Campine region after three years. Sci Total Environ 626:195–202
GB/T14848-2017 Chinese quality standard for groundwater. National standards of the People’s Republic of China
GB3838-2002 Environmental quality standards for surface water. National standards of the People’s Republic of China
Guo M (2016) Application of biochar for soil physical improvement. In: Guo M, He G , Uchimiya SM (Editors), Agricultural and environmental applications of biochar: advances and barriers. SSSA Special Publication. Soil Science Soc Amer, 677 S Segoe Rd, Madison, Wi 53711 USA, pp. 101-122
Hartley W, Dickinson NM, Riby P, Lepp NW (2009) Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environ Pollut 157:2654–2662
Hinsinger P, Marschner P (2006) Rhizosphere - perspectives and challenges - a tribute to Lorenz Hiltner 12-17 September 2004 - Munich, Germany. Plant Soil 283: VII-VIII
Irem S, Islam E, Maathuis FJM, Niazi NK, Li T (2019) Assessment of potential dietary toxicity and arsenic accumulation in two contrasting rice genotypes: Effect of soil amendments. Chemosphere 225:104–114
Jindo K, Mizumoto H, Sawada Y, Sanchez-Monedero MA, Sonoki T (2014) Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 11:6613–6621
Kuzyakov Y, Bogomolova I, Glaser B (2014) Biochar stability in soil: decomposition during eight years and transformation as assessed by compound-specific C-14 analysis. Soil Biol Biochem 70:229–236
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota - A review. Soil Biol Biochem 43:1812–1836
Leist M, Casey RJ, Caridi D (2000) The management of arsenic wastes: problems and prospects. J Hazard Mater 76:125–138
Lessl JT, Ma LQ (2013) Sparingly-soluble phosphate rock induced significant plant growth and arsenic uptake by Pteris vittata from three contaminated soils. Environ Sci Technol 47:5311–5318
Lessl JT, Luo J, Ma LQ (2014) Pteris vittata continuously removed arsenic from non-labile fraction in three contaminated-soils during 3.5 years of phytoextraction. J Hazard Mater 279:485–492
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O'Neill B, Skjemstad JO, Thies J, Luizao FJ, Petersen J, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730
Liang J, Yang Z, Tang L, Zeng G, Yu M, Li X, Wu H, Qian Y, Li X, Luo Y (2017) Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 181:281–288
Lim TJ, Spokas KA, Feyereisen G, Novak JM (2016) Predicting the impact of biochar additions on soil hydraulic properties. Chemosphere 142:136–144
Ma LQ, Komar KM, Tu C, Zhang WH, Cai Y, Kennelley ED (2001) A fern that hyperaccumulates arsenic (vol 409, pg 579, 2001). Nature 411:438–4U3
Mathews S, Ma LQ, Rathinasabapathi B, Natarajan S, Saha UK (2010) Arsenic transformation in the growth media and biomass of hyperaccumulator Pteris vittata L. Bioresour Technol 101:8024–8030
Matovic D (2011) Biochar as a viable carbon sequestration option: global and Canadian perspective. Energy 36:2011–2016
McGrath SP, Zhao FJ (2003) Phytoextraction of metals and metalloids from contaminated soils. Curr Opin Biotechnol 14:277–282
Mehlich A (1984) Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 15:1409–1416
Moreno-Jimenez E, Esteban E, Penalosa JM (2012) The fate of arsenic in soil-plant systems. In: Whitacre DM (ed) Reviews of Environmental Contamination and Toxicology, vol 215. Reviews of Environmental Contamination and Toxicology, pp 1–37
Niazi NK, Singh B, Van Zwieten L, Kachenko AG (2011) Phytoremediation potential of Pityrogramma calomelanos var. austroamericana and Pteris vittata L. grown at a highly variable arsenic contaminated site. Int J Phytoremediation 13:912–932
Niazi NK, Singh B, Van Zwieten L, Kachenko AG (2012) Phytoremediation of an arsenic-contaminated site using Pteris vittata L. and Pityrogramma calomelanos var. austroamericana: a long-term study. Environ Sci Pollut Res Int 19:3506–3515
Niazi NK, Bashir S, Bibi I, Murtaza B, Shahid M, Javed MT, Shakoor MB, Saqib ZA, Nawaz MF, Aslam Z, Wang H, Murtaza G (2016) Phytoremediation of arsenic-contaminated soils using arsenic hyperaccumulating ferns. In: Ansari AA, Gill SS, Gill R, Lanza GR, Newman L (eds) Phytoremediation: Management of Environmental Contaminants, vol 3. Springer International Publishing, Cham, pp 521–545
Ok YS, Chang SX, Gao B, Chung H-J (2015) SMART biochar technology-a shifting paradigm towards advanced materials and healthcare research. Environ Technol Innov 4:206–209
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180:169–181
Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49:643–668
Scally S, Davison W, Zhang H (2006) Diffusion coefficients of metals and metal complexes in hydrogels used in diffusive gradients in thin films. Anal Chim Acta 558:222–229
Shah AH, Shahid M, Khalid S, Natasha SZ, Bakhat HF, Murtaza B, Farooq A, Akram M, Shah GM, Nasim W, Niazi NK (2019) Assessment of arsenic exposure by drinking well water and associated carcinogenic risk in peri-urban areas of Vehari, Pakistan. Environ Geochem Health 41:1–13
Singh R, Singh S, Parihar P, Singh VP, Prasad SM (2015) Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol Environ Saf 112:247–270
Shi RY, Hong ZN, Li JY, Jiang J, Kamran MA, Xu RK, Qian W (2018) Peanut straw biochar increases the resistance of two Ultisols derived from different parent materials to acidification: A mechanism study. J Environ Manage 210:171–179
Sun Q, Chen J, Zhang H, Ding S, Li Z, Williams PN, Cheng H, Han C, Wu L, Zhang C (2014) Improved Diffusive Gradients in Thin Films (DGT) Measurement of total dissolved inorganic arsenic in waters and soils using a hydrous zirconium oxide binding layer. Anal Chem 86:3060–3067
Tan Z, Lin CSK, Ji X, Rainey TJ (2017) Returning biochar to fields: A review. Appl Soil Ecol 116:1–11
Tang J, Liao Y, Yang Z, Chai L, Yang W (2016) Characterization of arsenic serious-contaminated soils from Shimen realgar mine area, the Asian largest realgar deposit in China. J Soils Sediments 16:1519–1528
Tu S, Ma LQ, MacDonald GE, Bondada B (2004) Effects of arsenic species and phosphorus on arsenic absorption, arsenate reduction and thiol formation in excised parts of Pteris vittata L. Environ Exp Bot 51:121–131
USEPA (2007) Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils, vol. 3051A
Wang X, Ma LQ, Rathinasabapathi B, Liu Y, Zeng G (2010) Uptake and translocation of arsenite and arsenate by Pteris vittata L.: Effects of silicon, boron and mercury. Environ Exp Bot 68:222–229
Wang J, Zeng X, Zhang H, Li Y, Zhao S, Bai L, Su S, Wang Y (2018) Kinetic release of arsenic after exogenous inputs into two different types of soil. Environ Sci Pollut Res 25:12876–12882
Wu W, Yang M, Feng Q, McGrouther K, Wang H, Lu H, Chen Y (2012) Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 47:268–276
Xu R-k, A-z Z, J-h Y, Jiang J (2012) pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J Soils Sediments 12:494–502
Yan X, Zhang M, Liao X, Tu S (2012) Influence of amendments on soil arsenic fractionation and phytoavailability by Pteris vittata L. Chemosphere 88:240–244
Yang J, Yang J, Huang J (2017) Role of co-planting and chitosan in phytoextraction of As and heavy metals by Pteris vittata and castor bean – a field case. Ecol Eng 109:35–40
Ye W-L, Khan MA, McGrath SP, Zhao F-J (2011) Phytoremediation of arsenic contaminated paddy soils with Pteris vittata markedly reduces arsenic uptake by rice. Environ Pollut 159:3739–3743
Yin D, Wang X, Peng B, Tan C, Ma LQ (2017) Effect of biochar and Fe-biochar on Cd and As mobility and transfer in soil-rice system. Chemosphere 186:928–937
Yuan J-H, Xu R-K, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Zeng X, Xiao Z, Zhang G, Wang A, Li Z, Liu Y, Wang H, Zeng Q, Liang Y, Zou D (2018) Speciation and bioavailability of heavy metals in pyrolytic biochar of swine and goat manures. J Anal Appl Pyrolysis 132:82–93
Zhang H, Davison W (2015) Use of diffusive gradients in thin-films for studies of chemical speciation and bioavailability. Environ Chem 12:85–101
Zhang H, Zhao FJ, Sun B, Davison W, McGrath SP (2001) A new method to measure effective soil solution concentration predicts copper availability to plants. Environ Sci Technol 35:2602–2607
Zhang J-E, Ouyang Y, Ling D-J (2007) Impacts of simulated acid rain on cation leaching from the Latosol in south China. Chemosphere 67:2131–2137
Zhou H, Zhang D, Wang P, Liu X, Cheng K, Li L, Zheng J, Zhang X, Zheng J, Crowley D, van Zwieten L, Pan G (2017) Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: a meta-analysis. Agric Ecosyst Environ 239:80–89
Acknowledgement
This work was supported by National Natural Science Foundation of China (No.41977108), Fok Ying Tung Education Foundation for Young Teachers in Higher Education Institutions of China (No.151029), Hunan Young Talents Supporting Program (2017RS3032), and Cultivation Program for National Excellent Youth Science Foundation of Hunan Normal University (XP4180201).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Zheng, C., Wang, X., Liu, J. et al. Biochar-assisted phytoextraction of arsenic in soil using Pteris vittata L. Environ Sci Pollut Res 26, 36688–36697 (2019). https://doi.org/10.1007/s11356-019-06688-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06688-5